Translate this page into:
EPIYA (or -like) motifs in mammalian proteins
*Current address: Laboratory of Molecular Medicine Cell Biology, Graduate School of Biological Sciences, Nara Institute of Science and Technology, 8916-5, Takayama, Ikoma, Nara 630-0192, Japan. Tel.: +81 743725470 safarifatemeh@bs.naist.jp (Fatemeh Safari) safarif2003@gmail.com (Fatemeh Safari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 14 May 2014
Peer review under responsibility of King Saud University.

Abstract
Recently, two mammalian proteins with functional Glu-Pro-Ile-Tyr-Ala (EPIYA) or a similar sequence (EPIYA-like motif) have been identified. Pragmin contains a single EPIYA motif and p140Cap contains two EPIYA-like motifs (EPLYA/EGLYA). Searching the human proteome using the NCBI BLAST software revealed that EPIYA-like motifs such as EPLYA, ESIYE, EDLYA, ESIYA, EHIYD, EPIYD, ENIYE, EPVYA, EVVYA, and TPLYA (which are present in bacterial effectors and have roles in virulence) are present in mammalian proteins. Moreover, several of bacterial EPIYA-like motifs were duplicated in mammalian proteins. Since, only five mammalian proteins contain EPIYA motif, and there are no mammalian proteins with more than one copy of the EPIYA motif. We also showed that the most of EPIYA (or -like) motifs in mammalian proteins are predicted to be naturally ordered. Moreover, few mammalian proteins containing EPIYA (or -like) motif were tyrosine phosphorylated. On the other hand, most of the mammalian proteins contain “unfunctional EPIYA (or -like) motifs”. Notably, regulation of Src family kinases’ (SFKs) activity (including up and down regulation) originally is mediated by their own EPIYA like motifs (EPIYI and EPQYQ) and thereby, it seems that EPIYA (or -like) motifs are “potential regulators of SFKs”. Therefore, it can explain why EPIYA (or -like) motifs are restricted sites with low copy number in most mammalian proteins whereas in bacterial effector proteins, they can be universal sites with repetitive copies. In this study, we identified new mammalian proteins which contain functional EPIYA (or -like) motifs and thereby, their possible roles in cell signal transduction pathways. In this respect, functional experiments will be necessary to confirm our findings.
Keywords
EPIYA (or -like) motif
Mammalian proteins
SFKs
Bacterial effector proteins
1 Introduction
The presence of the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif or a sequence closely related to the EPIYA motif (EPIYA-like motif) in effector proteins that play roles in the virulence of pathogenic bacteria was analyzed (Table 1). Bacterial effector proteins enter mammalian cells through type III or type IV secretion systems (T3SS or T4SS), and these effector proteins are phosphorylated at tyrosine residues located at the EPIYA (or -like) motif by host kinases, which triggers an interaction with host cell SH2 domain-containing proteins and manipulates the function of host cells for more effective infection and improved colonization (Selbach et al., 2009; Backert et al., 2010; Hayashi et al., 2013).
Pathogen
Effector protein
EPIYA (or -like) motifs
Tyrosine kinases involved
References
H. pylori
CagA
EPIYA
SFKs, Abl
Poppe et al. (2007), Tammer et al. (2007), Mueller et al. (2012)
A. phagocytophilum
AnkA
ESIYE, EDLYA, ESIYA, EPIYA
SFKs, Abl
Ijdo et al. (2007)
C. trachomatis
Tarp
ENIYE
SFKs, Abl, Syk
Mehlitz et al. (2008)
H. ducreyi
LspA
EPIYG, EPVYA
SFKs
Deng et al. (2008)
B. henselae
Bep
EPLYA, EVVYA, TPLYA, EPLYA
SFKs (?)
Schulein et al. (2005)
EPEC; C. rodentium
Tir
VNPYA, EHIYD; EPIYD
Fyn, Abl
Campellone and Leong (2005), Phillips et al. (2004), Swimm et al. (2004), Deng et al. (2003)
Among mammalian proteins, it was demonstrated that Pragmin EPIYA motif undergoes tyrosine phosphorylation at the EPIYA motif by Src family kinases (SFKs) or in response to EGF stimulation. Tyrosine phosphorylation at the EPIYA allows Pragmin to interact with the SH2 domain of Csk, and thereby sequesters Csk in the cytoplasm. Sequestration of Csk by Pragmin has a positive feedback on SFK activity (Safari et al., 2011). In another study, Repetto et al. (2013) showed that N-terminal of p140Cap (also known as SRC kinase signaling inhibitor 1) contains two EPIYA-like motifs (EPLYA and EGLYA) which can be tyrosine phosphorylated at EPLYA and EGLYA by c-Abl or in response to integrin-mediated adhesion and EGF stimulation. Upon tyrosine phosphorylation, p140Cap EPLYA and EGLYA sequences serve as binding sites for the SH2 domain of Csk (Repetto et al., 2013; Sharma et al., 2013). Moreover, C-terminal of p140Cap contains a proline rich sequence which interacts with Src SH3 domain and thereby, facilitates inhibition of SFK activity by Csk (Di Stefano et al., 2007). Taken together, it has recently been proposed that the mammalian EPIYA (or -like) motif might have been exploited by pathogenic bacteria (Safari et al., 2011).
In this study, we identified all of bacterial EPIYA-like motifs in mammalian proteins and by using PhosphoSite, we explored the functional EPIYA (or -like) motifs in mammalian proteins. Furthermore, by using RONN tool, we did structure disorder prediction for mammalian proteins containing EPIYA (or -like) motifs. Our results provided new information about differences between mammalian and bacterial EPIYA (or -like) motifs. Furthermore, our findings may give insights into the role of EPIYA (or -like) motif in new mammalian proteins to regulate SFK activity as the target of EPIYA (or -like) motifs.
2 Material and methods
NCBI (National Center for Biotechnology Information, U.S. National Library of Medicine, www.ncbi.nlm.nih.gov) was used to obtain sequences of mammalian EPIYA (or -like) motif containing proteins.
PhosphoSitePlus was used for the detection of tyrosine phosphorylation at EPIYA (or -like) containing proteins (http://www.phosphosite.org) (Hornbeck et al., 2012).
RONN tool was used for prediction of structure disorder of EPIYA (or -like) motif in mammalian proteins, (www.strubi.ox.ac.uk/RONN) (Yang et al., 2009).
WebLogo 3 was used to align and display protein sequences (http://code.google.com/p/weblogo/) (Crooks et al., 2004).
3 Results
3.1 Tyrosine phosphorylation and structure disorder prediction of EPIYA segments in mammalian proteins
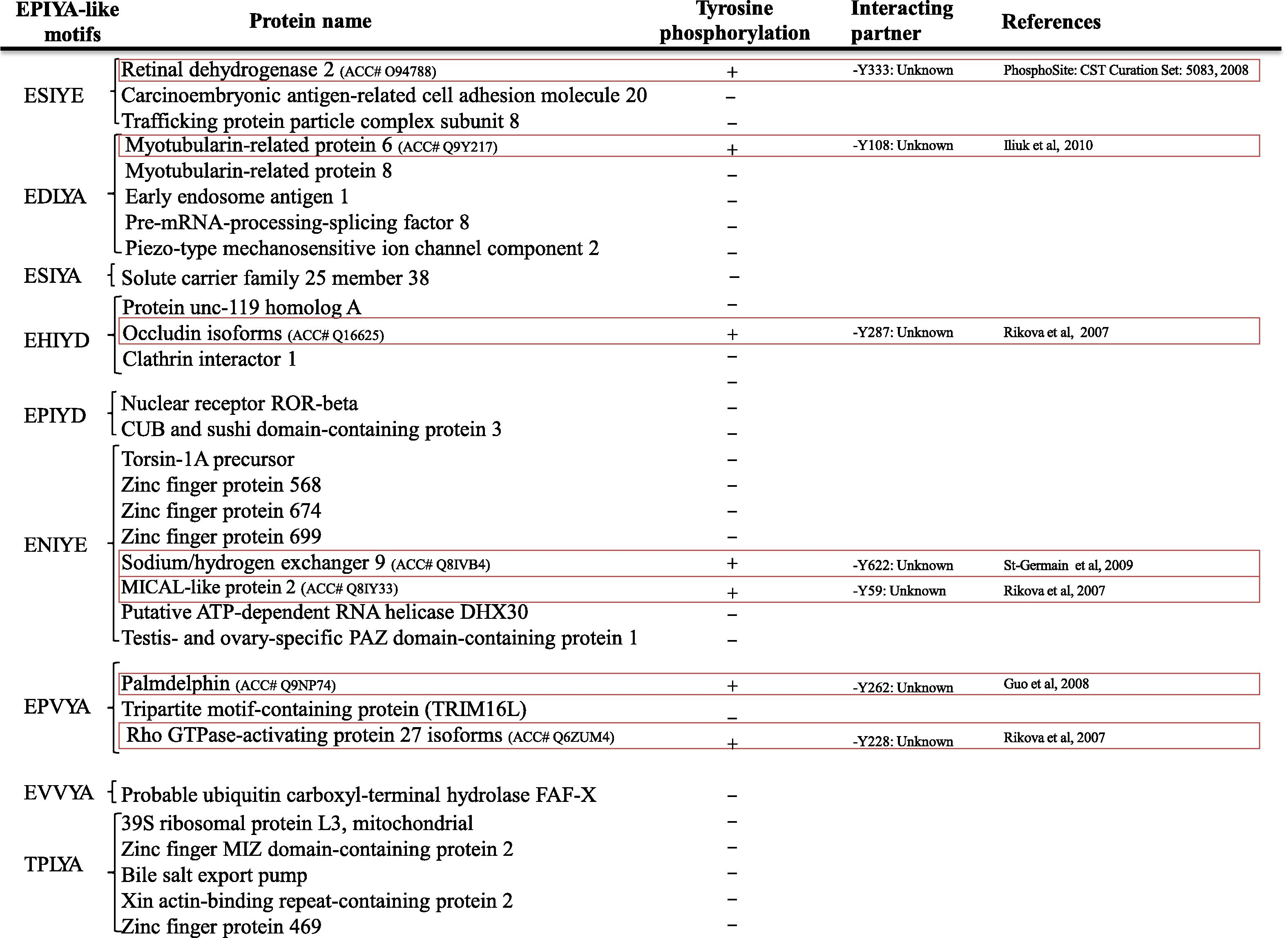
It was assumed that flexible feature of bacterial EPIYA (or -like) segments allows them to interact with huge number of SH2-domain containing proteins in a tyrosine-phosphorylation-dependent manner (Hayashi et al., 2012, 2013). Moreover, it was shown that five mammalian proteins contain EPIYA motif (Safari et al., 2011). Therefore, the question was whether EPIYA segments in mammalian proteins show order feature which is different from flexible feature of bacterial EPIYA segment and thereby, by using Phosphosite, we first explored tyrosine phosphorylation of EPIYA motif mammalian containing proteins. Our results showed that two out five mammalian proteins containing EPIYA motif were tyrosine phosphorylated (Fig. 1A). We then used RONN tool to predict disorder structure of EPIYA segments of mammalian proteins which were tyrosine phosphorylated. Our results indicated that Pragmin EPIYA segment revealed flexible feature which is similar to that of bacterial EPIYA segment (Fig. 1B). This result was not unexpected because of similar sequences distal between Pragmin EPIYA motif (EPIYAESAKR) and CagA-B EPIYA motif (EPIYAQVAKK).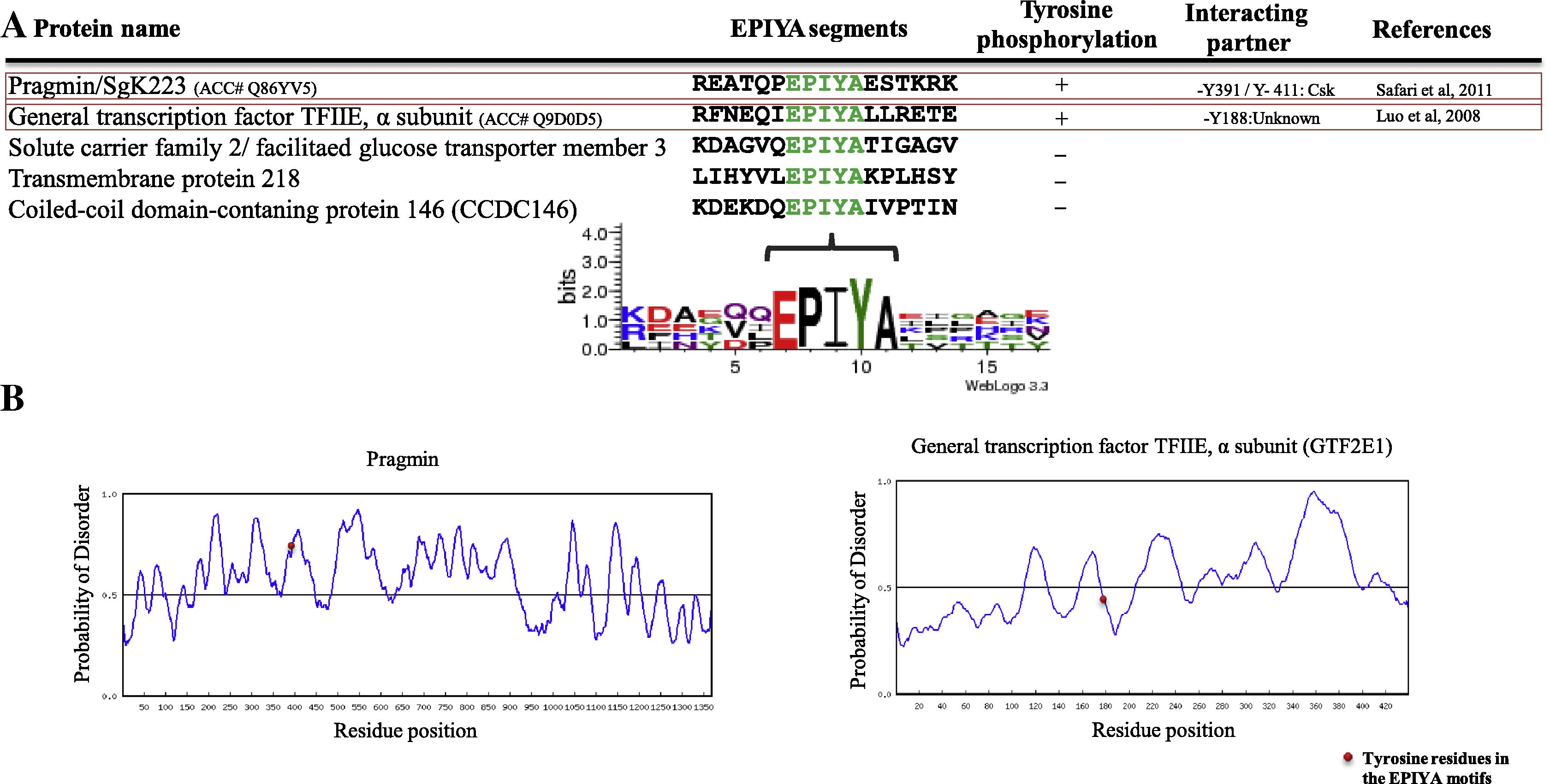
(A) List of human EPIYA containing proteins. By using PhosphoSite, the functional EPIYA motifs containing proteins were found which are shown in red boxes. The logo plot was also generated with the WebLogo tool. (B) Structure disorder prediction of mammalian proteins which contain functional EPIYA motif calculated with the RONN tool. (See above-mentioned references for further information.)
3.2 Identification of functional EPIYA-like motifs in mammalian proteins
p140Cap contains two EPIYA-like motifs (EPLYA and EGLYA) (Repetto et al., 2013). This result indicates that EPIYA-like motif in mammalian proteins has a function similar to that of EPIYA motif. Moreover, it raises a possibility that the EPIYA-like motifs (which are present in bacterial effectors and have roles in virulence) are present in mammalian proteins. To examine this possibility, first, we searched EPIYA-like motifs (EPLYA or EGLYA) in the human proteome by using NCBI BLAST software and our results showed that 11 mammalian proteins contain single EGLYA sequences and two mammalian proteins contain single EPLYA sequences (Fig 2A and B). However, only two out 14 mammalian proteins containing EGLYA or EPLYA were tyrosine phosphorylated. Disorder structure prediction for EPIYA-like segments of two mammalian proteins indicated that p140Cap has rigid feature and the other protein (Partitioning defective 3 homolog B) showed flexible feature. Interestingly, sequences distal Partitioning defective 3 homolog B EGLYA (EGLYAKVNKP) showed homology with CagA-A EPIYA motif (EPIYAKVNKK) (Fig. 2A). In this respect, it was shown that EPIYA like motifs such as ESIYE, EDLYA, ESIYA, EPIYG, VNPYA, EHIYD, EPIYD, ENIYE, EPVYA, EVVYA and TPLYA, which have roles in virulence, can be found in bacterial effector proteins (Selbach et al., 2009; Backert et al., 2010; Hayashi et al., 2013). We then investigated whether mammalian proteins contain such EPIYA-like motifs. Our results revealed that except EPIYG and VNPYA, all bacterial EPIYA-like motifs are present in mammalian proteins (Table 2), and bacterial EPIYA-like motif (ENIYE) is more common in mammalian proteins (Table 3). Among mammalian proteins containing bacterial EPIYA (or -like) motifs, only seven proteins were tyrosine phosphorylated. Disorder structure prediction of seven mammalian proteins containing bacterial EPIYA-like motifs showed that four proteins are naturally disordered (Fig. 3).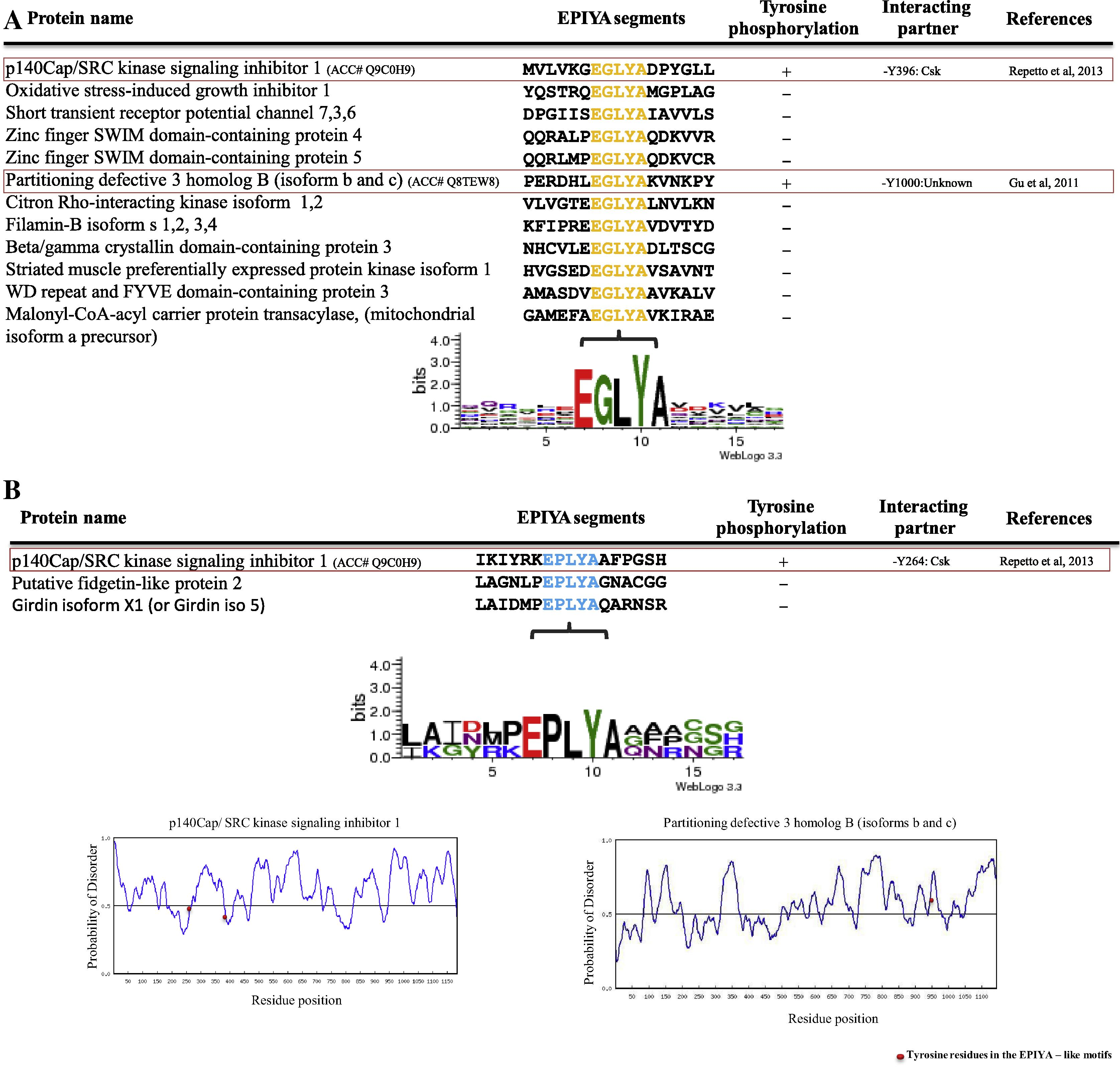
(A and B) List of human EPIYA-like motifs (EGLYA and EPLYA) containing proteins. Functional EGLYA and EPLYA motifs in mammalian proteins are shown in red boxes. Alignment of sequence surrounding EGLYA and EPLYA motifs is displayed and structure disorder prediction of mammalian proteins which contain functional EPIYA-like motifs (EPLYA or EGLYA) is shown. (See above-mentioned references for further information.)
EPIYA-like motif
Frequencies
EPLYA
3
ESIYE
3
EDLYA
5
ESIYA
1
EPIYG
0
VNPYA
0
EHIYD
3
EPIYD
2
ENIYE
8
EPVYA
3
EVVYA
1
TPLYA
5
∗EGLYA
11
Total
45
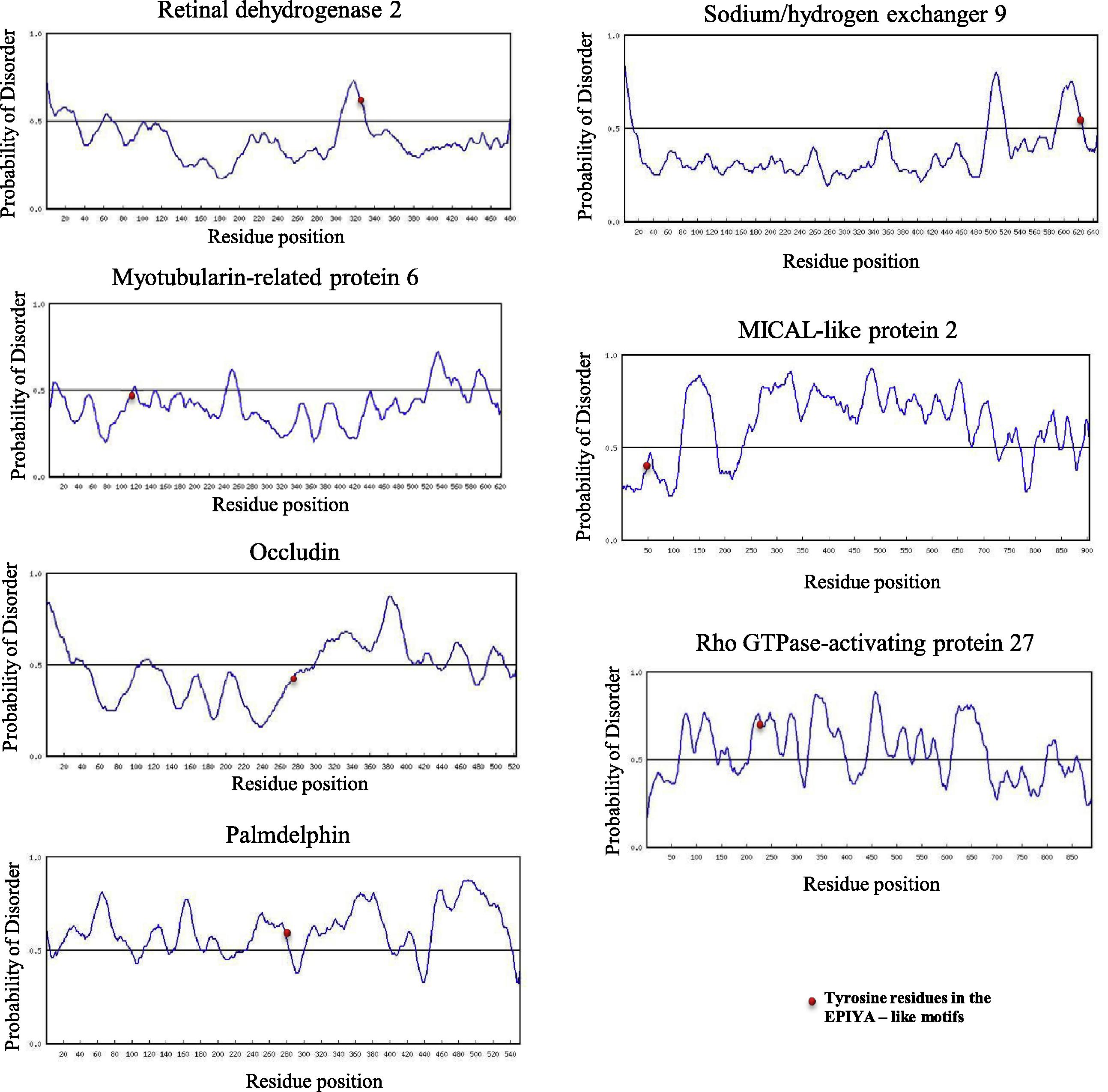
Structure disorder prediction of mammalian proteins which contain functional EPIYA-like motifs.
3.3 Identification of mammalian proteins with duplication of bacterial EPIYA like motifs
It was assumed that the presence of numerous EPIYA (or -like) copies in bacterial effector proteins with disordered features allowed them to interact with several SH2-domain-containing proteins (Hayashi et al., 2012, 2013). The presence of two EPIYA like motifs (EPLYA and EGLYA) in p140Cap, raises a possibility that the mammalian proteins may contain duplication of EPIYA like motifs and we wished to know the structure disorder prediction of them. So, we explored human proteome to find all proteins with duplication of bacterial EPIYA-like motifs and we found that several bacterial EPIYA-like motifs are duplicated in mammalian proteins (eight proteins) and only two of them were tyrosine phosphorylated (Fig. 4A). However, none of them are predicted to be disordered in EPIYA-like segments (Fig. 4B).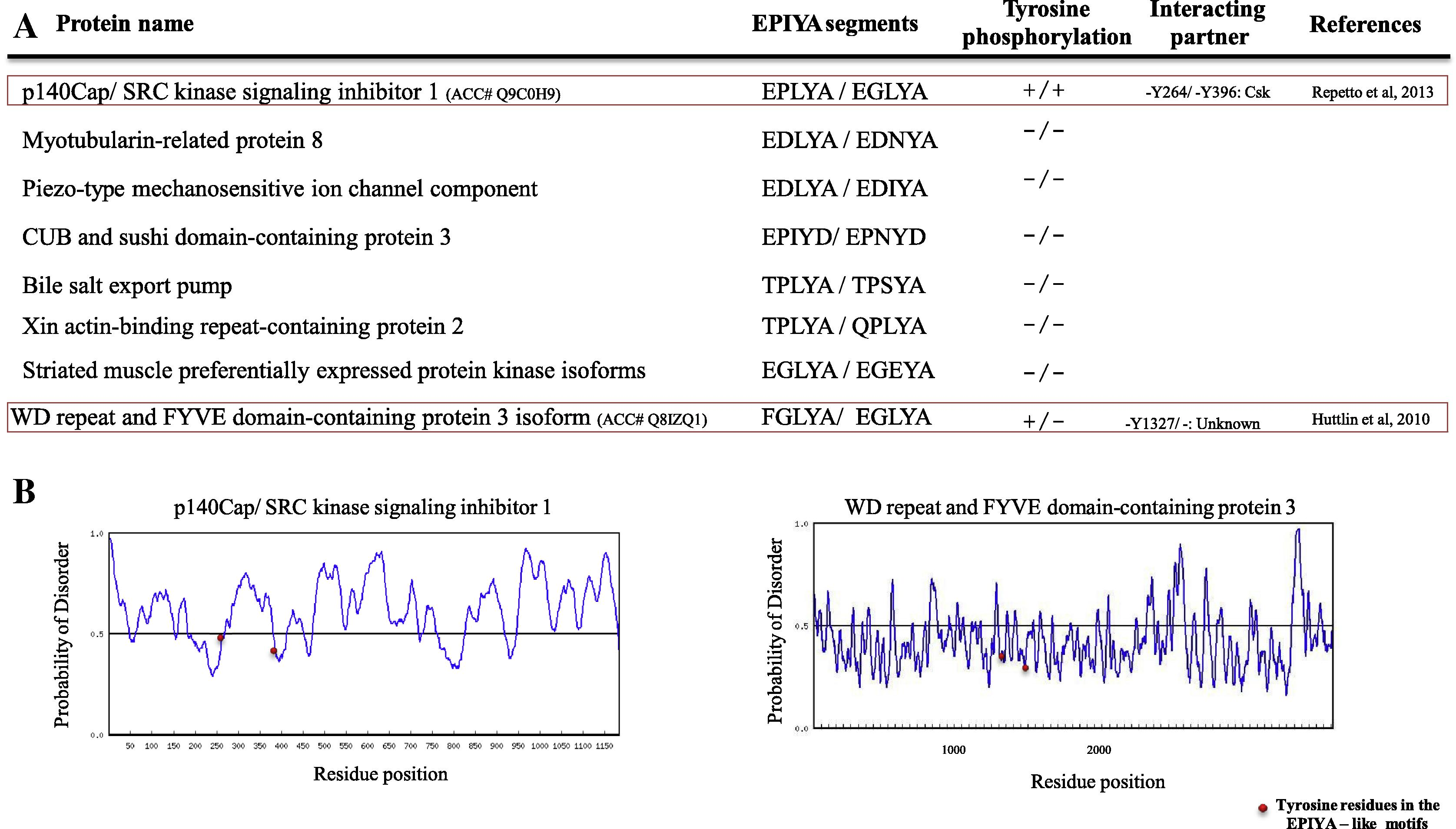
(A) List of human proteins which contain double EPIYA-like motif. (B) Structure disorder prediction of mammalian proteins with double EPIYA like motifs. (See above-mentioned references for further information.)
3.4 The presence of functional EPIYA-like motifs in SFK members and regulation of SFK activity via their own EPIYA-like motifs
In the current study, we focused on the functional EPIYA (or -like) motifs (that have virulence roles in bacterial effector proteins) in mammalian proteins. However, it was shown that p140Cap contains EGLYA sequence that is not present in bacterial effector proteins. Therefore, it suggests that the non-bacterial EPIYA-like motifs are also present in mammalian proteins. In this respect, we found that members of Src family kinases contain duplication of functional EPIYA-like motifs. It was shown that N-terminal SH2 domain of SHP-1 interacts with phosphorylated tyrosine residue (or-Y338) which is located at EPIYA-like motif,” EPIYI”, of c-Src and thereby, up regulates Src activity in platelets and lymphocytes (Smart et al., 1981; Somani et al., 1997). Moreover, Y338 is conserved in all SFK members, although, the function of this tyrosine residue in the other SFK members is still unknown (Fig. 5A). Of note, the activity of SFKs was down regulated by Csk via EPIYA-like motif “EPQYQ” (Okada et al., 1988a,b; Okada et al., 1991). On the other hand, Csk is the responsible kinase which phosphorylates tyrosine residue (or-Y530 in c-Src) that is located at EPIYA-like motif “EPQYQ” (Fig. 5B). These results suggest that EPIYA (or -like) motifs can originally play regulatory roles for SFK members.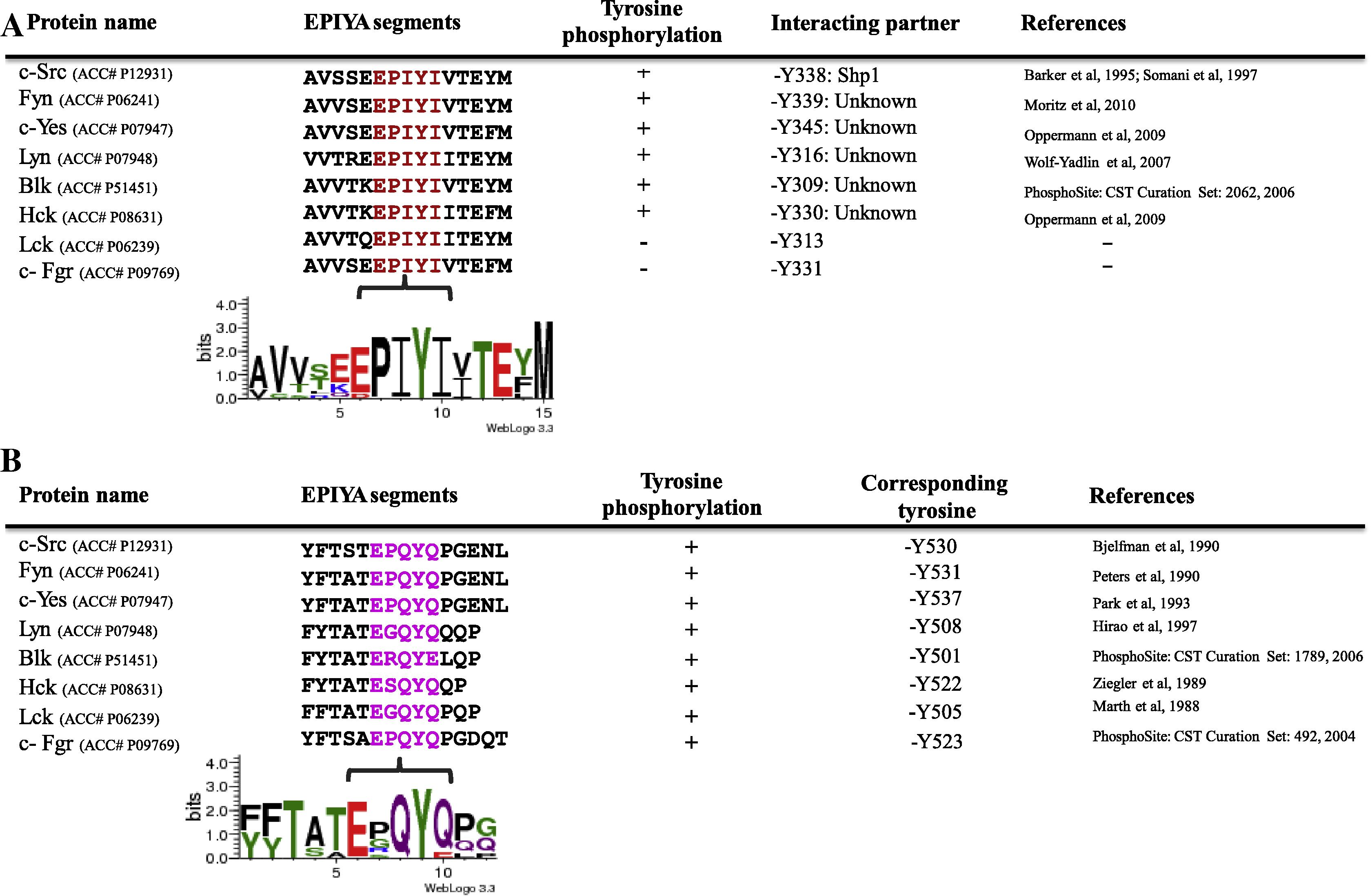
(A and B) The presence of two EPIYA-like motifs (EPIYI and EPQYQ) in SFK members. (See above-mentioned references for further information.)
4 Conclusion
The sequence EPxYAxV (where x is any amino acid) is significantly underrepresented in mammalian proteomes (Selbach et al., 2009). However, the number of mammalian proteins containing EPIYA-like motifs is not as low as it was proposed before (Xu et al., 2010; this paper). This difference may be due to explanation of “EPIYA-like motif”. In the current study, we identified 50 mammalian proteins which contain bacterial EPIYA (or -like) motifs. Our results showed that most of mammalian proteins containing EPIYA (or -like) motifs were not tyrosine phosphorylated at EPIYA (or -like) motifs and thereby, they are “unfunctional EPIYA (or -like) motifs”. Since, bacterial effector proteins containing same EPIYA (or -like) motifs were tyrosine phosphorylated. In this regard, we found that out of 11 functional EPIYA (or -like) motifs in mammalian proteins, six proteins were predicted to be naturally unfolded. Moreover, out of 39 proteins containing unfunctional EPIYA (or -like) motif, 32 proteins were predicted to be ordered at EPIYA (or -like) segments. Therefore, in mammalian proteins containing EPIYA (or -like) motif, tyrosine phosphorylation at unfolded EPIYA (or -like) segments is more frequent than ordered EPIYA (or -like) segments. Although, bacterial effector proteins were continuously tyrosine phosphorylated by host tyrosine kinases which seems to be due to unfolded nature of bacterial EPIYA (or -like) motifs. Our results also raise a possibility that EPIYA (or -like) motifs can be potential regulators of SFK members in the cell machinery. On the other hand, SFK activity can be directly regulated via their own EPIYA-like motifs (EPIYI and EPQYQ) or via indirect feedback by functional EPIYA (or -like) motifs containing proteins (including mammalian proteins and pathogenic effector proteins). During long-term co-evolution with hosts, pathogenic bacteria enable to employ host key regulators such as EPIYA (or -like) to better colonization. These key regulators were expanded via recombination (Furuta et al., 2011; Majazki et al., 2013). However, in the mammalian hosts, such key regulators are mostly unfunctional with low copy number. Furthermore, it seems that intramolecular interactions in mammalian proteins containing EPIYA (or -like) allowed the EPIYA (or -like) segments to be as unaccessed sites. Notably, SFKs have pleiotropic functions in the regulation of fundamental cellular processes. SFKs are highly conserved across the animal kingdom, and regulation of SFKs is a prerequisite for evolution of multicellular animals (Segawa et al., 2006; Okada, 2012). Our findings showed that via tyrosine phosphorylation at EPIYA (or -like) motifs, mammalian proteins can interact with Csk (Safari et al., 2011; Repetto et al., 2013) and SHP-1 (Somani et al., 1997). In this study, several mammalian proteins with functional EPIYA (or -like) motifs were identified for which interacting partner(s) are still unknown. So, it would be interesting to gain a better understanding of SFK activity via EPIYA (or -like) motifs. Moreover, elucidation of the structure of mammalian proteins containing EPIYA (or -like) motif proteins will provide more information on the specificity of mammalian EPIYA (or -like) segments. It is known that SFKs are activated in some human cancers, including colon and breast cancer (Summy and Gallick, 2003). In this respect, the role of Pragmin EPIYA motif in activation of SFKs should be explored. Notably, SFKs are candidates for the treatment of human cancers (Kopetz et al., 2007; Hollande et al., 2010). Therefore, the specificity of EPIYA (or -like) segments to regulation of SFK activity should be further explored.
Acknowledgment
We would like to thank the Reviewers for their valuable comments and suggestions.
References
- The versatility of helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15:163-176.
- [Google Scholar]
- Characterization of pp60c-src tyrosine kinase activities using a continuous assay: autoactivation of the enzyme is an intermolecular autophosphorylation process. Biochemistry. 1995;34:14843-14851.
- [Google Scholar]
- Early activation of endogenous pp60src kinase activity during neuronal differentiation of cultured human neuroblastoma cells. Mol. Cell. Biol.. 1990;10:361-370.
- [Google Scholar]
- Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol. Microbiol.. 2005;56:416-432.
- [Google Scholar]
- Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol.. 2003;48:95-115.
- [Google Scholar]
- Haemophilus ducreyi LspA proteins are tyrosine phosphorylated by macrophage-encoded protein tyrosine kinases. Infect Immun.. 2008;76:4692-4702.
- [Google Scholar]
- P140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J.. 2007;26:2843-2855.
- [Google Scholar]
- Evolution of cagA oncogene of Helicobacter pylori through recombination. PLoS One. 2011;6:e23499.
- [Google Scholar]
- Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6:e15640.
- [Google Scholar]
- Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl. Acad. Sci. U.S.A.. 2008;105:692-697.
- [Google Scholar]
- Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012;12:20-33.
- [Google Scholar]
- Bacterial EPIYA effectors – where do they come from? What are they? Where are they? Where are they going? Cell. Microbiol.. 2013;15:377-385.
- [Google Scholar]
- Translocation of the Csk homologous kinase (Chk/Hyl) controls activity of CD36-anchored Lyn tyrosine kinase in thrombin-stimulated platelets. EMBO J.. 1997;16:2342-2351.
- [Google Scholar]
- The long road to colorectal cancer therapy: searching for the right signals. Drug Resist. Updat.. 2010;13:44-56.
- [Google Scholar]
- PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res.. 2012;40:D261-D270.
- [Google Scholar]
- A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174-1189.
- [Google Scholar]
- Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell. Microbiol.. 2007;9:1284-1296.
- [Google Scholar]
- In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Mol. Cell. Proteomics. 2010;9:2162-2172.
- [Google Scholar]
- Src continues aging: current and future clinical directions. Clin. Cancer Res.. 2007;13:7232-7236.
- [Google Scholar]
- Global impact of oncogenic Src on a phosphotyrosine proteome. J. Proteome Res.. 2008;7:3447-3460.
- [Google Scholar]
- Anaplasma phagocytophilum strains from voles and shrews exhibit specific ankA gene sequences. BMC Vet. Res.. 2013;9:235.
- [CrossRef] [Google Scholar]
- Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol. Cell. Biol.. 1988;8:540-550.
- [Google Scholar]
- Complex kinase requirements for Chlamydia trachomatis Tarp phosphorylation. FEMS Microbiol. Lett.. 2008;289:233-240.
- [Google Scholar]
- Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci. Signal.. 2010;3:ra64.
- [CrossRef] [Google Scholar]
- C-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. Clin. Invest.. 2012;122:1553-1566.
- [Google Scholar]
- Protein tyrosine kinase in rat brain: neonatal rat brain expresses two types of pp60c-src and a novel protein tyrosine kinase. J. Biochem.. 1988;104:297-305.
- [Google Scholar]
- Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem. Biophys. Res. Commun.. 1988;154:796-802.
- [Google Scholar]
- CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem.. 1991;266:24249-24252.
- [Google Scholar]
- Large-scale proteomics analysis of the human kinome. Mol. Cell. Proteomics. 2009;8:1751-1764.
- [Google Scholar]
- C-Yes tyrosine kinase activity in human colon carcinoma. Oncogene. 1993;8:2627-2635.
- [Google Scholar]
- In vivo phosphorylation and membrane association of the fyn proto-oncogene product in IM-9 human lymphoblasts. Oncogene. 1990;5:1313-1319.
- [Google Scholar]
- Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat. Cell Biol.. 2004;6:618-625.
- [Google Scholar]
- Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462-3472.
- [Google Scholar]
- Mapping of p140Cap phosphorylation sites: the EPLYA and EGLYA motifs have a key role in tyrosine phosphorylation and Csk binding, and are substrates of the Abl kinase. PLoS One. 2013;8:1-12.
- [Google Scholar]
- Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190-1203.
- [Google Scholar]
- Mammalian Pragmin regulates Src family kinases via the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif that is exploited by bacterial effectors. Proc. Natl. Acad. Sci. U.S.A.. 2011;108:14938-14943.
- [Google Scholar]
- A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. U.S.A.. 2005;102:856-861.
- [Google Scholar]
- Functional development of Src tyrosine kinases during evolution from a unicellular ancestor to multicellular animals. Proc. Natl. Acad. Sci. U.S.A.. 2006;103:12021-12026.
- [Google Scholar]
- Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbes. 2009;5:397-403.
- [Google Scholar]
- Identification of two regions in the p140Cap adaptor protein that retain the ability to suppress tumor cell properties. Am. J. Cancer Res.. 2013;3:290-301.
- [Google Scholar]
- Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp6Ovsrc) and its normal cellular homologue (pp6oc rc) Proc. Natl. Acad. Sci. U.S.A.. 1981;78:6013-6017.
- [Google Scholar]
- Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem.. 1997;272:21113-21119.
- [Google Scholar]
- Multiple myeloma phosphotyrosine proteomic profile associated with FGFR3 expression, ligand activation, and drug inhibition. Proc. Natl. Acad. Sci. U.S.A.. 2009;106:20127-20132.
- [Google Scholar]
- Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev.. 2003;22:337-358.
- [Google Scholar]
- Enteropathogenic E. coli use redundant tyrosine kinases to form actin pedestals. Mol. Biol. Cell.. 2004;15:3520-3529.
- [Google Scholar]
- Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132:1309-1319.
- [Google Scholar]
- Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc. Natl. Acad. Sci. U.S.A.. 2007;104:5860-5865.
- [Google Scholar]
- Effector prediction in host-pathogen interaction based on a Markov model of a ubiquitous EPIYA motif. BMC Genomics. 2010;11(Suppl. 3):S1.
- [Google Scholar]
- RONN: the bio-basis function neural network technique applied to the detection of natively disordered regions in proteins. Bioinformatics. 2009;21:3369-3376.
- [Google Scholar]
- Transformation of NIH 3T3 fibroblasts by an activated form of p59hck. Mol. Cell Biol.. 1989;9:2724-2727.
- [Google Scholar]