Translate this page into:
Structurally diverse alkaloids from Tecomella undulata G. Don flowers
*Corresponding author. Tel.: +92 22 9213429 30; fax: +92 22 9213431 shahabuddinmemon@yahoo.com (Shahabuddin Memon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 5 March 2014
Peer review under responsibility of King Saud University.

Abstract
The present study is aimed to focus on the extraction and screening of alkaloids from the flowers of Tecomella undulata G. Don. It has been observed that the plant is very rich in alkaloids and the modified method employed for the extraction of alkaloids is efficient and selective, where the interference of other secondary metabolites is negligible. The identification of each compound was made through gas chromatography–mass spectrometry (GC–MS). A total of eleven (11) structurally diverse alkaloids were identified for the first time from the flowers of this plant. The present study may be helpful in the fields of natural products’ chemistry and pharmaceuticals as well as drug discovery science and technology.
Keywords
Tecomella undulata
Extraction
GC–MS
Natural products
Alkaloids
1 Introduction
Tecomella undulata G. Don belongs to the family Bignoniaceae, locally known as Rohira in Sindh (Laghari et al., 2013) and is used in traditional medication system (Jain et al., 2012). It is widely distributed in Tharparkar and Rajasthan Deserts of Pakistan and India (Chal et al., 2011). In indigenous system of medicine, it is used against spleen, liver and abdominal complaints (Parekh and Chanda, 2007). The plant possesses many pharmacological activities such as anti-inflammatory, analgesic potential, antibacterial, antioxidant and hepatoprotective activity against thioacetamide-induced hepatotoxicity (Ahmad et al., 1994; Parekh et al., 2005; Laghari et al., 2013; Khatri et al., 2009). The phytochemical studies report that the plant contains flavonoids (Taneja et al., 1975), iridoid glucosides (Joshi et al., 1975; Verma et al., 1986), alkaloids, saponins (Hungund and Pathak, 1971) and it also contains anti-HIV agents (Azam, 1999).
Alkaloids are very useful pharmaceutical agents because of their biological activities (Gotti et al., 2006; Kumar et al., 2009) such as antimicrobial (Deng et al., 2011), antioxidant (Benabdesselam et al., 2007), analgesic potential and anti-inflammatory activities (Chen et al., 2012). T. undulata also possesses same activities as those of alkaloids. Consequently, it is quite reasonable to attribute that those biological activities are due to the presence of alkaloids. Moreover, due to its usage as a raw plant there may be some toxic substances mostly present in plants for their defense (Laghari et al., 2012), which may be harmful to human health. Because, generally beside other bioactive constituents; few alkaloids are toxic to health (Clement et al., 1997, 1998; Radulović et al., 2012); therefore, there must be a proper profile for the alkaloids that are present in this plant. Previously, no work has been reported as a systematic study regarding the alkaloid profile of T. undulata except preliminary confirmation of alkaloids via phytochemical studies (Hungund and Pathak, 1971). Thus, in the present study it was aimed to focus on the screening of alkaloids in flowers among the active constituents of this plant, which are responsible to cure the diseases and provide a proper alkaloid profiling, because this plant as a whole is used in traditional medication system for a long time.
2 Experimental
2.1 Chemicals
In this study, methanol, ethanol, chloroform, and n-hexane (Analytical grade) were purchased from a dealer of Lab-Scan Analytical Sciences, Bangkok, Thailand. Hydrochloric acid, sodium sulfate (anhydrous) and ammonium hydroxide (Sigma–Aldrich, Taufkirchen, Germany) were used.
2.2 Plant material
The flowers of T. undulata were collected in March 2010 from a small village Rana Bajeer district Mithi at Tharparkar of Sindh province, Pakistan. The plant species was identified by a plant taxonomist, in the Institute of Plant Sciences, University of Sindh Jamshoro. A voucher specimen (T. undulata 15176) has been deposited in the herbarium of the institute. Collected plant material was shade dried, coarsely powdered and used for further studies.
2.3 Extraction of alkaloids
Extraction of alkaloids was carried out by following a reported method (Berkov et al., 2005) with some modifications. Briefly 20 g of dried and powdered flowers of T. undulata were soaked in ethanol (200 mL) for 48 h. The plant material was filtered, the filtrate was collected and ethanol was evaporated in vacuum by using a rotary evaporator at 40 °C. The obtained extract (0.5 g i.e. 2.5% w/w) was dissolved in 200 mL hydrochloric acid (1 M) and shaken vigorously on a vortex mixer. After this, it was extracted twice with n-hexane (15 × 2 mL) in a separating funnel in order to separate out fatty or non-polar contents. The remaining aqueous portion was made basic (ca. pH 10) with ammonium hydroxide solution (10%). Finally, this basic solution was extracted three times with chloroform (15 × 3 mL) and organic phase was washed twice with distilled water (20 × 2 mL) and then dried in anhydrous sodium sulfate (5 g). The alkaloid fraction (0.05 g i.e. 0.25% w/w) was obtained by evaporating the chloroform that was dissolved in methanol (10 mL). The presence of alkaloids was confirmed by Dragendroff’s reagent that showed a brownish-red precipitate, which is a sign of the presence of alkaloids (Parekh and Chanda, 2007). At last, the alkaloids’ fraction was analyzed on GC–MS for further studies.
2.4 Gas chromatography–mass spectrometry (GC–MS) analysis of alkaloids
The methanol fraction of alkaloids (0.05 g/10 mL) was injected (1 μL) into gas chromatograph (Shimadzu 2010, Singapore 118227) by using an auto injector (PAL Switzerland) connected with it. For separation of alkaloids, DB-5 MS capillary column (60 m, 0.25 mm i.d., 0.25 μm) was used by running helium gas (99.999%) and temperature programming of column oven was set as; initial temperature 50 °C, which was increased by a ramp of 10 °C per min up to 240 °C. As per the standard operating procedure (SOP) of instrument supplier, injector and interface (GC and MS) temperature was set at 250 °C, i.e. 10 °C higher to final temperature of column oven. MS conditions were; mass range was optimum (i.e. 35–650 Dalton), while scan events 50 and temperature at 200 °C. The resulting total ion chromatogram (Fig. 1) was observed in GC solution software post run analysis option and compounds responsible for each peak were confirmed by matching their mass fragmentation patterns to the National institute of standard technology (NIST) library.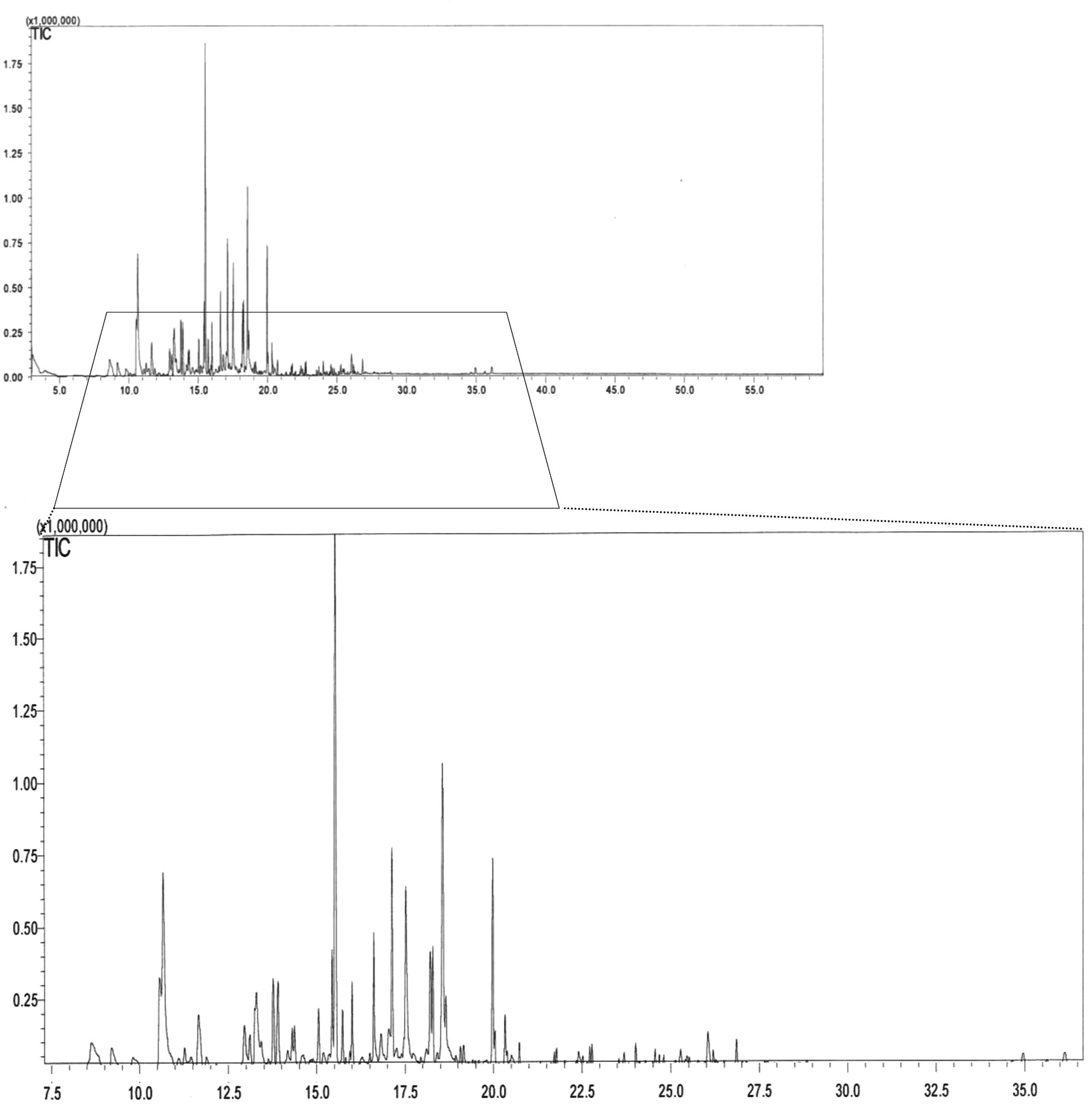
Gas chromatogram comprised of total alkaloids identified from the flowers' extract of Tecomella undulata.
3 Results and discussion
In this study, alkaloids’ extraction was carried out and it is the first time that a systematic analysis of alkaloids present in the flowers of T. undulata G. Don is reported. It has been found that the extract of T. undulata flowers is very rich in alkaloids that were initially confirmed by Dragendroff’s reagent. In the modification of method n-hexane was used instead of chloroform to remove fatty constituents and considerable reduction of interfering compounds was observed. Thus, most of alkaloids were extracted with a minor quantity of other constituents by this modified extraction method explained above. Later on, identification of alkaloids was made by direct sampling to GC–MS without any derivatization. A total of 11 alkaloids were identified, i.e. 2-pyrrolidinemethanol (1), 3-amino-4-pyrazolecarbonitrile (2), 3-(1-methyl-2-pyrrolidinyl)pyridine (3), 2-methyl-6-propylpiperidine (4), 1-piperidineethanol (5), 4-formyl-1,3-dihydro-1,3-dimethyl-2H-imidazole-2-thione (6), 5-acetylpyrimidine-2,4,6(1H,3H,5H)-trione (7), 1-(1-cyclohexen-1-yl)Pyrrolidine (8), decahydroquinoline (9), 5,7-dimethyl-1,3-diazadamantan-6-one (10), and 2,4-dihydro-5-methyl-2-phenyl-3H-Pyrazol-3-one (11) (Table 1). Total alkaloids’ percentage in methanolic extracts of alkaloid fraction by GC–MS analysis was found to be 49.39%. Further confirmation of the alkaloids was made by comparing their m/z and mass fragmentation patterns with the NIST library (Fig. 2). All peaks of GC–MS chromatogram were processed for similarity search index and it was found that each of those was matching ⩾95% to their respective standard compounds.
Compound No.
Name of compound
Retention time (min)
Peak area%
Molecular mass (m/z)
Fragments
1.
2-Pyrrolidinemethanol
11.65
2.63
101
70, 43
2.
3-Amino-4-pyrazolecarbonitrile
13.28
2.63
108
79, 63
3.
3-(1-Methyl-2-pyrrolidinyl)pyridine
13.76
2.73
162
133, 84
4.
2-Methyl-6-propylpiperidine
13.90
2.92
141
126, 98
5.
1-Piperidineethanol
15.05
1.44
129
98
6.
4-Formyl-1,3-dihydro-1,3-dimethyl-2H-imidazole-2-thione
15.53
16.63
156
128
7.
5-Acetylpyrimidine-2,4,6(1H,3H,5H)-trione
15.99
1.97
170
155
8.
1-(1-Cyclohexen-1-yl) pyrrolidine
16.61
2.87
151
136,123
9.
Decahydroquinoline
17.13
4.82
139
96
10.
5,7-Dimethyl-1,3-diazadamantan-6-one
17.52
5.94
180
138
11.
2,4-Dihydro-5-methyl-2-phenyl-3H-Pyrazol-3-one
19.97
4.81
174
105, 91, 77
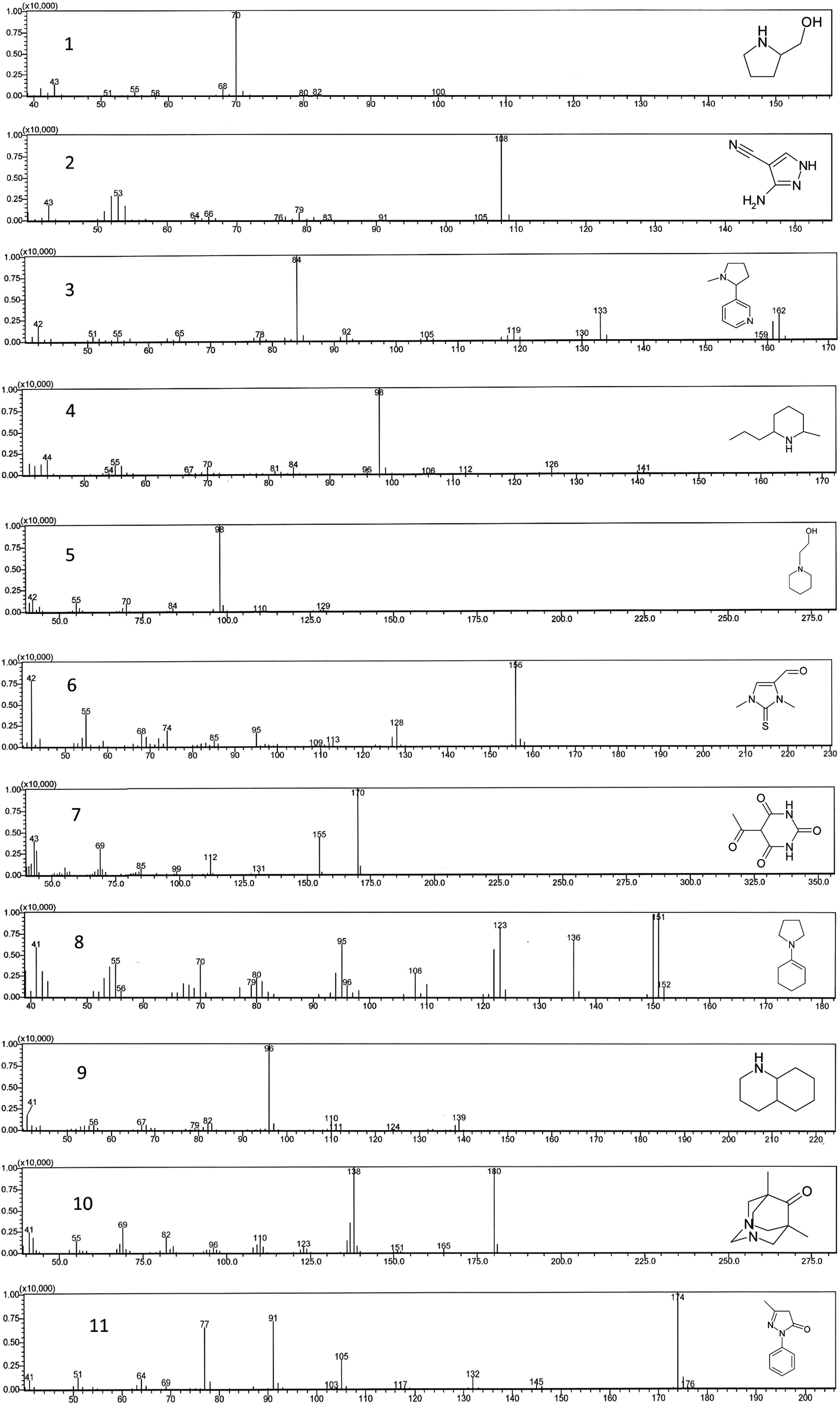
GC–MS total ion chromatogram observed for the alkaloids, showing their m/z and fragmentation patterns and their chemical structures.
It is a general observation that in species of any genus of plants, alkaloids of similar type, i.e. different derivatives of same type are usually found. However, this trend was not observed in this plant. It contains structurally diverse alkaloids, i.e. cyclic, bicyclic and aromatic. All alkaloids identified from this plant are different in nature and they are not derivatives of each other. It is in agreement with the previously reported literature (Rivière et al, 2011; Angiosperm Phylogeny Group, 2009) stating that the family Bignoniaceae has the capability to produce structurally diverse secondary metabolites. This unique behavior of possessing various types of alkaloids is very important and makes T. undulata more valuable in the field of medicinal chemistry.
4 Conclusions
It could be concluded that T. undulata is highly rich in alkaloids and diversity in their structures was observed. All eleven alkaloids are reported for the first time from this plant. The extraction method used in this study is efficient to extract alkaloids with minor quantity of other compounds. The chromatographic method used in this study is reproducible and efficient to separate all alkaloids in a single run and well resolved peaks are observed. Present profiling of alkaloids may be helpful to the pharmaceutical formulations and folklore practitioners using this plant as a source of medicine. The data presented here may be useful and informative to those who are working in pharmaceutical industries.
Acknowledgments
Support during the present work made by the National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro/Pakistan and Pakistan Council of Scientific and Industrial Research, Karachi/Pakistan is gratefully acknowledged.
References
- Preliminary screening of methanolic extracts of Celastrus paniculatus and Tecomella undulata for analgesic and anti-inflammatory activities. J. Ethnopharmacol.. 1994;42:193-198.
- [Google Scholar]
- Angiosperm Phylogeny Group, 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 161, 105–121.
- Anti-HIV agents and other compounds from Tecomella undulata. Orient. J. Chem.. 1999;15:375-377.
- [Google Scholar]
- Antioxidant activities of alkaloid extracts of two Algerian species of Fumaria: Fumaria capreolata and Fumaria bastardii. Rec. Nat. Prod.. 2007;1:28-35.
- [Google Scholar]
- CGC–MS of alkaloids in Leucojum aestivum plants and their in vitro cultures. Phytochem. Anal.. 2005;16:98-103.
- [Google Scholar]
- A phytopharmacological overview on Tecomella undulata G. Don. J. Appl. Pharma. Sci.. 2011;1:11-12.
- [Google Scholar]
- Analgesic and anti-inflammatory activity and pharmacokinetics of alkaloids from seeds of Strychnos nux-vomica after transdermal administration: effect of changes in alkaloid composition. J. Ethnopharmacol.. 2012;139:181-188.
- [Google Scholar]
- Toxic amines and alkaloids from Acacia berlandieri. Phytochemistry. 1997;46:249-254.
- [Google Scholar]
- Toxic amines and alkaloids from Acacia rigidula. Phytochemistry. 1998;49:1377-1380.
- [Google Scholar]
- Antimicrobial activity of extract and two alkaloids from traditional Chinese medicinal plant Stephania dielsiana. Food Chem.. 2011;124:1556-1560.
- [Google Scholar]
- Analysis of Amaryllidaceae alkaloids from Narcissus by GC–MS and capillary electrophoresis. J. Pharm. Biomed. Anal.. 2006;42:17-24.
- [Google Scholar]
- Hungund, B.L., Pathak, C.H., 1971. A survey of plants in Gujarat, India, for alkaloids, saponins, and tannins, U.S.D.A. Forest service research paper NE-201.
- Traditional uses, phytochemistry and pharmacology of Tecomella undulata – a review. Asian Pac. J. Trop. Biomed. 2012:S1918-S1923.
- [Google Scholar]
- 6-O-veratryl catalposide, a new iridoid glucoside from Tecomella undulata. Phytochemistry. 1975;14:1441-1442.
- [Google Scholar]
- Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharmacol.. 2009;122:1-5.
- [Google Scholar]
- Biological activity of alkaloids from Solanum dulcamara L. Nat. Prod. Res.. 2009;23:719-723.
- [Google Scholar]
- Antifungal ursene type triterpene from roots of Alhagi camelorum. Helv. Chem. Acta. 2012;95:1556-1560.
- [Google Scholar]
- Tecomella undulata G. Don: a rich source of flavonoids. Ind. Crops Prod.. 2013;43:213-217.
- [Google Scholar]
- In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol.. 2007;31:53-58.
- [Google Scholar]
- Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol.. 2005;29:203-210.
- [Google Scholar]
- A novel toxic alkaloid from poison hemlock (Conium maculatum L., Apiaceae): identification, synthesis and antinociceptive activity. Food Chem. Toxicol.. 2012;50:274-279.
- [Google Scholar]
- Chemotaxonomic interest of iridoids isolated from a Malagasy species: Perichlaena richardii. Biochem. Syst. Ecol.. 2011;39:797-825.
- [Google Scholar]
- Chemical constituents of flowers of Tecomella undulata. Indian J. Chem.. 1975;13:427-428.
- [Google Scholar]
- Structure of undulatin: a new iridoid glycoside from Tecomella undulata. Planta Med.. 1986;52:359-362.
- [Google Scholar]







