Translate this page into:
Cytotoxic and apoptosis induction potential of Mimusops elengi L. in human cervical cancer (SiHa) cell line
*Corresponding author. Tel.: +91 9443630839, +91 416 2202359; fax: +91 416 2243092 ncsarada@vit.ac.in (N.C. Sarada)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 30 October 2013
Peer review under responsibility of King Saud University.

Abstract
Bullet wood tree, also called Spanish cherry (Mimusops elengi L.) is a medium-sized evergreen tree belonging to family Sapotaceae which is widely used in the treatment of different ailments. The present study investigated cytotoxic potential of methanolic leaf and bark extracts against human cervical cancer cell line (SiHa) by MTT (3-(4,5-dimethylthiazol-2-yl)-2; 5-diphenyltetrazolium bromide) assay, the apoptotic cells were quantified further by flow cytometry by FACS fluorescence activated cell sorting. The extract of M. elengi bark and leaf was effective towards tested cell line, with IC50 values of 35.08 ± 2.92 μg/ml and 67.46 ± 4.21 μg/ml respectively. Further there is an increase in apoptotic bodies from 0.24% to 60% and 69% after treatment with extracts. These extracts exhibit significant cytotoxic effect by inducing apoptosis. The M. elengi has never shown anticancer activity on SiHa cells. These findings suggested that extracts and compounds from this plant could be useful for preventing and treating human gynaecologic cancer disease.
Keywords
Mimusops elengi
Cytotoxic
Caspase-3
Apoptosis
Cervical cancer
1 Introduction
Cervical cancer constitutes the second most common cancer in women. This is due to the infection with Human Papilloma Virus (HPV), notably type 16 and 18 virus (Alvarez-Salas and DiPaolo, 2007). Although the mortality is high, it is preventable through screening and treatment of precancerous lesion and the use of vaccines (Berrington and Lall, 2012). Conventional cancer therapies like radiotherapy and chemotherapy will damage the cancer cells and also affect some healthy cells in the body and cause side effects like nausea, emesis, bone marrow problems, hair loss, fatigue etc., and there are no prophylactic measures available to address the side effects (Flay and Matthews, 1995), therefore natural products are being tested for the treatment but these are yet to prove their efficacy in preclinical and clinical studies.
Recent studies have shown that antioxidants and herbal derivatives may be effective against prevailing HPV infection (Bharti et al., 2009; Su et al., 2011; Wu et al., 2006). Plants remain as an important and continuing source for treating cancers. More than 70% of the 177 anticancer drugs approved since 1950 were natural products or mimetics, and the research still continues (Brower, 2008).
Apoptosis is a process of programmed cell death and is a central event essential to maintain tissue homeostasis for all organ systems in the human body (Kumar et al., 2012). Inhibition of apoptosis results in a cell proliferation, such as cancer. Tumour cells use a variety of molecular mechanisms to suppress apoptosis (Subhadradevi et al., 2011); hence the induction of apoptosis in tumour cells is believed to be a specific therapeutic approach towards cancer chemotherapy.
Spanish cherry (Mimuosops elengi L., Sapotaceae) is a small to large evergreen tree distributed throughout the greater parts of India. In ayurveda, the bark, flowers, fruit and seeds are having cardiotonic, alexipharmic, stomachic, astringent cooling, anthelmintic, tonic, and febrifuge properties (Mitra, 1981). The bark and fruits of this plant are used in the treatment of diarrhoea and dysentery (Jahan et al., 1995). Rinsing mouth with bark decoction is believed to strengthen the gums, reduce inflammation, prevent bleeding of gums, and to stop bad breath caused by pyorrhoea and dental caries (Baliga et al., 2011). Different parts of the plant have also been reported for anti microbial (Ali et al., 2008), anti ulcer (Payal et al., 2003), anti anxiety (Gayatri et al., 2011), anti oxidant, anti hyperglycaemic (Ganu et al., 2010), anti hyperlipidemic, anti helminthic (Gadamsetty et al., 2013), anti inflammatory and anti pyretic properties (Purnima et al., 2010). Several triterpenoids, steroids, steroidal glycosides, flavonoids and alkaloids have been identified and reported from this plant (Misra and Mitra, 1968).
Cervical cancer is a major health issue where chemotherapy is not effective due to its side effects, thus the use of pharmacological agents that are active towards HPV cancer cells with lesser side effects is strongly needed. This study is an attempt to evaluate apoptotic effects of methanolic extracts of leaves and bark of M. elengi on human cervical cancer cells.
2 Materials and Methods
2.1 Chemicals and reagents
Commercially available MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Sigma (St Louis, MO). Caspase-3 apoptosis kit was purchased from BD Pharmingen, USA. All other reagents and solvents were of analytical grades.
2.2 Preparation of extracts
Fresh leaves and stem bark were collected in January 2010 in the city of Vellore (12°58′ North, 79°09′ East), India. The plant parts were identified and authenticated by using various taxonomic markers and other available literature by consulting a botanist in National Institute of Herbal Science, Chennai, India. The herbarium samples were prepared and stored at our laboratory for future reference. The specimens were dried under shade, pulverized and extracted (60 g) with 400 ml hexane to remove chlorophyll and oily substances and followed by methanol at 70 °C using soxhlet extraction. The extracts were concentrated to dryness at 50 °C under reduced pressure using a rotary evaporator (Buchi, Switzerland). The extracts were kept at below 4 °C until further use (Kumar et al., 2012).
2.3 Preliminary phytochemical screening
Preliminary phytochemical screening of methanolic extracts of M. elengi leaves and bark was done for the presence of alkaloids, anthraquinones, amino acids, carbohydrates, flavonoids, saponins, steroids, tannins, and phenols by employing standard screening tests (Harbone, 1998).
2.4 Evaluation of cytotoxic activity
2.4.1 Cell culture
The HPV16 positive human cervical cancer cell line (SiHa) was obtained from the American Type Culture Collection (ATCC No. HTB-35), USA and checked for PCR positivity and contaminations. SiHa cells were cultured in a sterile T75 flask containing DMEM medium supplemented with fetal bovine serum (10% v/v) at 37 °C in a humidified atmosphere of 95% air and 5% CO2 incubator.
2.4.2 Cytotoxicity assay
The Cytotoxicity effects of methanolic extracts of leaf and bark against SiHa were determined by MTT dye uptake method (Mahata et al., 2012). The SiHa cells (1 × 103) seeded and grown overnight in 96-well plate, were treated with plant extracts at a concentration ranging from 1 to 100 μg/ml for 24 h at 37 °C in a CO2 incubator. Two hours prior to the completion of treatment 0.025 ml of MTT solution (5 mg/ml in PBS solution) was added to microplates and incubated at 37 °C. After incubation lysis buffer (20% SDS and 50% dimethyl formamide) was added to each well and then incubated overnight at 37 °C for solubilization of formazan crystals. Absorbance was measured at 570 nm with the aid of a 96-well multi-scanner autoreader (Biotek, Winooski, Vermont) with the lysis buffer serving as blank. The percentage of cell viability was calculated using the formula:
Percentage cell viability = {OD of the experiment samples/OD of the control} × 100.
2.5 Flow cytometric analysis of apoptotic cell death by quantitation of Caspase-3
The activity of caspase 3 was measured using the active caspase 3 apoptosis kit (BD Pharmingen, USA) following the manufacturer’s protocol. Briefly, 50 μg of the extract was incubated with SiHa cells for 12 h and harvested. The attached and detached cells were pooled and pelleted with centrifugation at 200xg for 5 min at 4 °C (Mahata et al., 2011). The cells were permeabilized, fixed and stained for active caspase 3 (PE-conjugated). The cells were then analysed by fluorescence activated cell sorting (FACS). The number of 10,000 events was acquired and the cells were properly gated for analysis using FACSAria instrument equipped with Flowjo software (Bravo-Cuellar et al., 2010).
2.6 Data analysis
Results of triplicate experiments were analysed using Microsoft Excel 2007 and presented as mean ± SD (n = 3).
3 Results
3.1 Preliminary phytochemical analysis
The preliminary phytochemical screening of methanol extracts showed abundant amount of saponins and flavonoids and trace amounts of carbohydrates and anthraquinones in both leaf and bark extracts. Amino acids and tannins were absent in both the extracts.
3.2 Cytotoxic activity
In SiHa cell lines after treatment with appropriate concentrations of plant extracts, the cell viability was tested after 24 h by MTT assay. The results of both the extracts are summarised in Fig 1, which showed a dose dependant activity towards the SiHa cell line. The results are demonstrated as percentage viability and represented as mean ± standard deviation (n = 3). From the results, the methanolic extract of M. elengi bark and leaf showed promising cytotoxic activity towards SiHa cells with IC50 values of 35.08 ± 2.92 μg/ml and 67.46 ± 4.21 μg/ml respectively.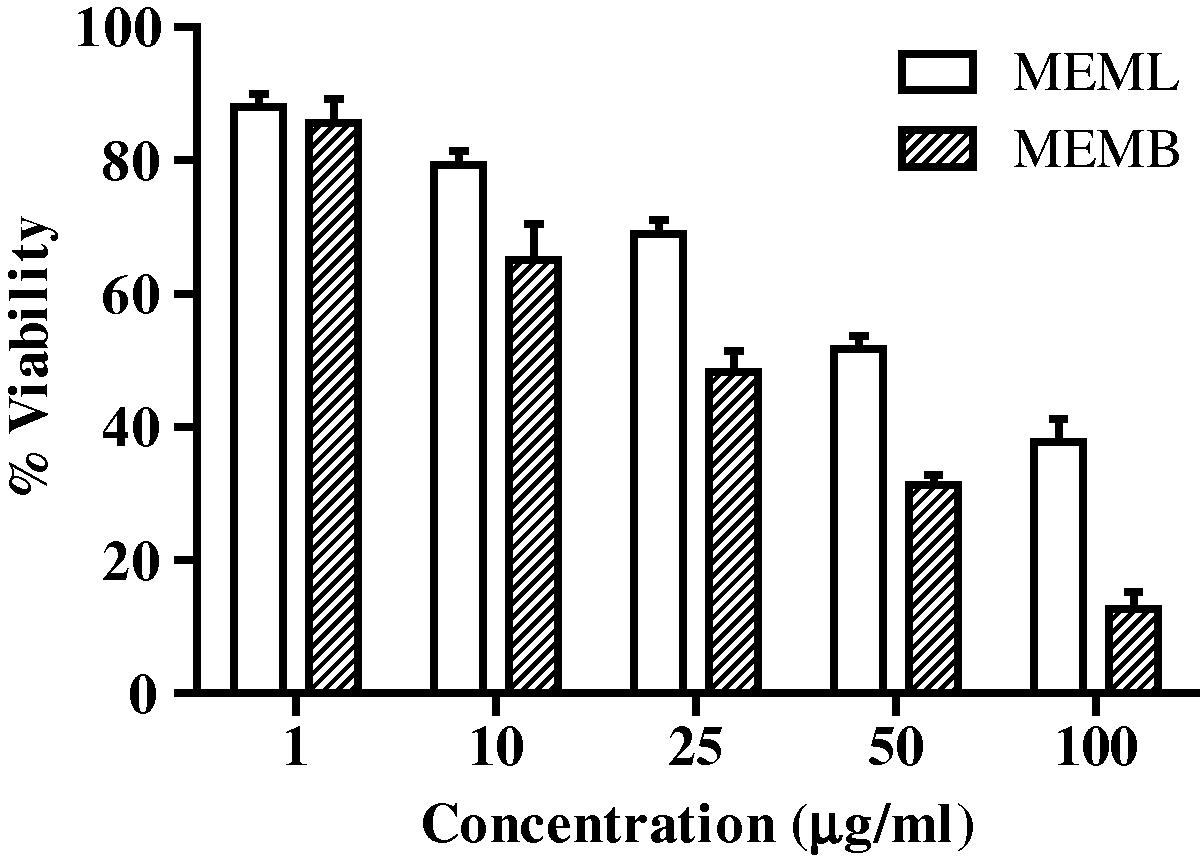
Cytotoxic activity of methanolic extract of M. elengi leaves (MEML) and methanoilic extract of M. elengi bark (MEMB) against Cervical cancer cell lines (SiHa) Values are Mean ± SD (n = 3).
3.3 Quantitation of caspase-3 activity by flow cytometric analysis
In order to confirm the apoptotic effect of the extract the activation of caspase-3 by flow cytometric analysis was done. Treatment of SiHa cell lines with methanolic extract of M. elengi at a concentration of 50 μg/ml resulted in the expression of caspase-3. The expressed caspase-3 cells were quantified flow cytometrically and the results are summarised in Fig. 2. The result showed a significant increase of caspase-3 levels after treatment of test extracts.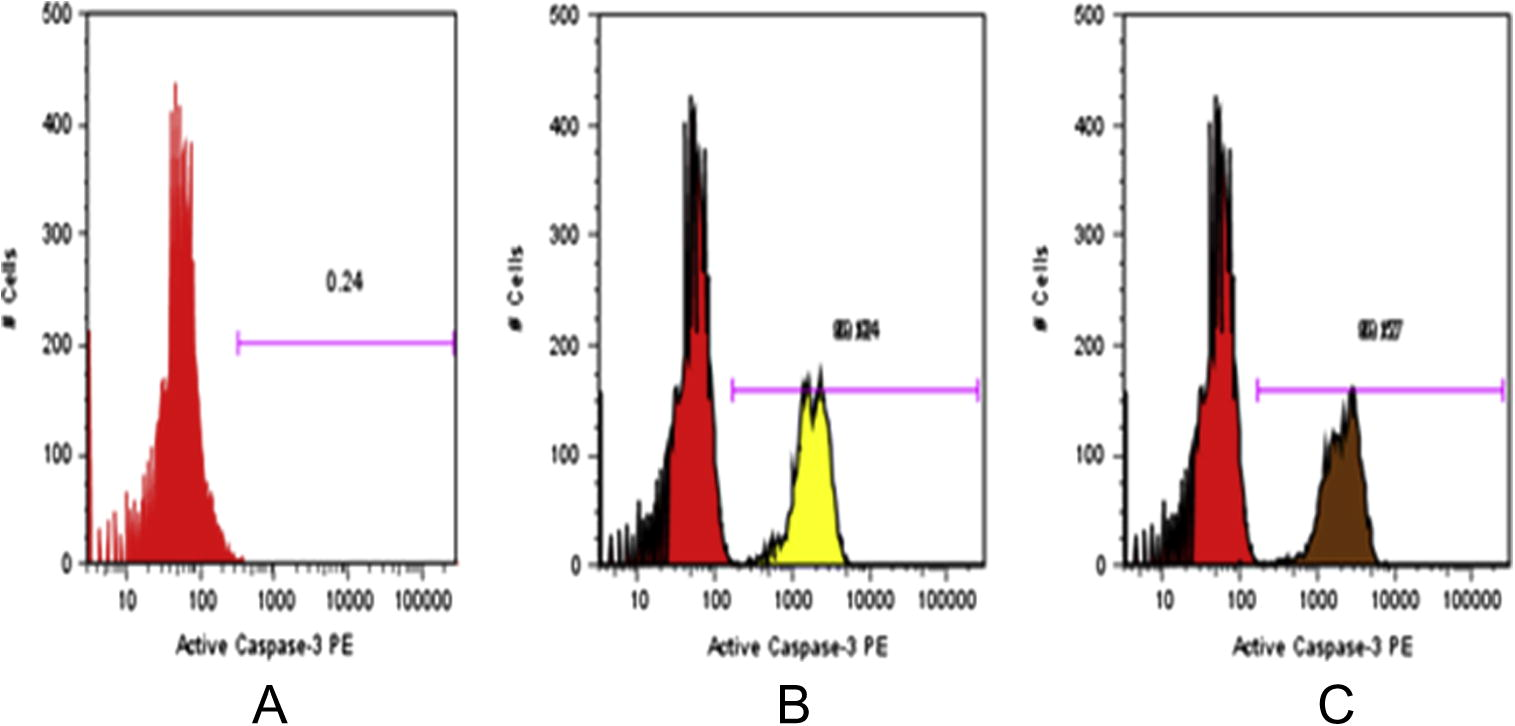
Flow Cytometric analysis of activation of Caspase-3 in cervical cancer cell lines (SiHa). (A), control untreated cells. (B), cells treated with MEML. (C), cells treated with MEMB.
4 Discussion
Cervical cancer is a major problem worldwide, reporting about 15% of neoplasms (Um et al., 2002). Current treatments include chemotherapy, radiotherapy and surgical ablation which show less satisfactory results, thus there is a need for safer and effective alternative treatment for different kinds of cancers.
Cytotoxicity test is mainly carried out to screen the cytotoxic potentiality of the compounds present in the extracts which affect basic cellular functions. Cytotoxic potential of the sample was measured by MTT dye uptake method, where active cells convert the water soluble MTT to an insoluble purple formazan, further solubilised and its concentration was determined (Liu et al., 1997). We found that the extracts of M. elengi bark and leaf were significantly toxic to SiHa cells with IC50 values 35.08 ± 2.92 and 67.46 ± 4.21 μg/ml respectively, when compared to the control. The bark extract was more toxic than leaf towards SiHa cell line. Cytotoxic activity of M. elengi is reported earlier as well, where Bhujbal et al. (2011) reported in vitro cytotoxic activity of methanolic bark extracts of M. elengi by A. cepa root tip assay. Bark extract was found to significantly decrease the percentage mitotic index and root length. In another study, Kar et al. (2012) reported the anti tumour activity of leaves of M. elengi against Ehrlich’s ascites carcinoma treated mice. Hence, the results imply that the methanolic extracts of leaf and bark were toxic towards tumour cells.
Apoptosis plays a major role in the regulation of tissue homeostasis and elimination of abnormal cells. Apoptosis is believed to be deregulated in cancer. An ideal therapeutic goal for cancer is to trigger apoptotic death selectively in tumour cells. Most of the antitumour drugs kill the cancer cells by stimulating the apoptotic pathway (Evan and Vousden, 2001). The key biochemical event involved in the induction of apoptosis is the activation of caspase-3 which is mediated through proteolytic cleavage of procaspase-3 via upstream caspases (caspase 7/9 or caspase 8). Activity of the caspase-3 which is an effect of caspases involved in both mitochondrial (extrinsic) as well as death receptor (intrinsic) pathways of apoptosis was expected in drug induced cytotoxicity of cancer cells. Therefore, drugs that restore the apoptotic pathways have the potential for effective treatment of tumours (Gao et al., 2011). To provide further evidence supporting the involvement of apoptosis, the caspase-3 was quantified by flow cytometry. Treatment of SiHa cell lines with methanolic extracts of leaves and bark of M. elengi at a concentration 50 μg/ml, for 12 h resulted in an increase of expression of caspase-3 from 0.24% to 69% and 60% for bark and leaf extracts, respectively. Thus it proves that the cytotoxic cell death is due to apoptosis. So the extract possibly induces caspase-3 and promotes apoptosis.
A number of bioactive molecules including flavonoids, phenoic compounds, alkaloids, and terpenoids previously reported for their cancer properties were identified from this plant in several studies (Gami et al., 2012). Among those isolated bioactive molecules, Lupeol, betulinic acid, gallic acid, and taraxerol have been shown in several reports to possess anti-cancer activity (Shoeb, 2006; Swain et al., 2012). In addition, many number of bioactive molecules were isolated and identified from the leaf and bark of this plant (Misra and Mitra, 1968). Therefore, the anti-cancer activity observed in crude leaf and bark extracts may be specifically due to any single chemical compound or it may due to phytochemical constituents. According to the results obtained, M. elengi leaf and bark extracts appeared to be potent anti-cancer agent and to the best of our knowledge this is the first report on anti-cancer activity of M. elengi against SiHa cell line.
In conclusion, the present study demonstrates the cytotoxic properties of methanolic extracts of leaf and bark of M. elengi L. Further studies to characterise the active principles and to elucidate the mechanism of action are in progress.
References
- An evaluation of antimicrobial activities of Mimusops elengi Linn. Res. J. Agric. Biol. Sci.. 2008;4:871-874.
- [Google Scholar]
- Molecular approaches to cervical cancer therapy. Curr. Drug. Discov. Technol.. 2007;4:208-219.
- [Google Scholar]
- Chemistry and medicinal properties of the Bakul (Mimusops elengi Linn): a review. Food Res. Int.. 2011;44:1823-1829.
- [Google Scholar]
- Anticancer activity of certain herbs and spices on the cervical epithelial carcinoma (HeLa) cell line. Evidence-based complementary and alternative medicine 2012
- [CrossRef] [Google Scholar]
- Anti-human papillomavirus therapeutics: facts & future. Indian J. Med. Res.. 2009;130(3):296-310.
- [Google Scholar]
- Evaluation of cytotoxic activity of barks of Mimusops elengi. Eurasia J. Biosci.. 2011;5:73-79.
- [Google Scholar]
- Bravo-Cuellar, A., Ortiz-Lazareno1, P.C., Lerma-Diaz1, J.M., Dominguez-Rodriguez1, J.R., JaveSuarez1, L.F., Aguilar-Lemarroy1, A., Toro-Arreola, S.D., et al., 2010. Sensitization of cervix cancer cells to Adriamycin by Pentoxifylline induces an increase in apoptosis and decrease senescence. Mol. Cancer. 9, 114.
- Back to nature: extinction of medicinal plants threatens drug discovery. JNCI J. Natl. Cancer. Inst.. 2008;100(12):838-839.
- [Google Scholar]
- The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys.. 1995;31:399-404.
- [Google Scholar]
- Phytochemical analysis and in-vitro anthelmintic activity of Mimusops elengi Linn and Drypetes sepiaria. Int. J. Pharm. Pharm. Sci.. 2013;5(1):126-128.
- [Google Scholar]
- Ethnobotanical, phytochemical and pharmacological review of Mimusops elengiLinn. Asian Pacific J. Trop. Biomed.. 2012;2(9):743-748.
- [Google Scholar]
- Antioxidant and Antihyperglycemic potential of methanolic extract of bark of Mimusops elengi in mice. Res. J. Pharm. Biol. Chem. Sci.. 2010;1:67-77.
- [Google Scholar]
- Tumor cell selective cytotoxicity and apoptosis induction by an herbal preparation from Brucea javanica. N. Am. J. Med. Sci. (Boston). 2011;4(2):62-66.
- [Google Scholar]
- Anti-anxiety activity of Mimusops elengi barks extract in experimental animals. Res. J. Pharm. Biol. Chem. Sci.. 2011;2:405-410.
- [Google Scholar]
- Phtochemical methods (third ed.). London: Chapman & Hall; 1998.
- Evaluation of antitumor activity of Mimusops elengi leaves on Ehrlich’s ascites carcinoma-treated mice. J. Diet. Suppl.. 2012;9(3):166-177.
- [Google Scholar]
- Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pacific J. Cancer Prev.. 2012;13(6):2729-2734.
- [Google Scholar]
- Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem.. 1997;69(2):581-593.
- [Google Scholar]
- Berberine modulates AP-1 activity to uppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer. 2011;10:39.
- [Google Scholar]
- Mahata, S., Maru, S., Shukla, S., Pandey, A., Mugesh, G., Das, B. C., Bharti, A.C., 2012. Anticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complementary and Alternative Medicine. 12, 15.
- Constituents of leaves, hardwood and root of Mimusops elengi. Phytochemsitry. 1968;7:501-502.
- [Google Scholar]
- Bakula- A reputed drug of Ayurveda, its history, uses in Indian medicine. Indian J. Hist. Sci.. 1981;12:169-180.
- [Google Scholar]
- Study of Mimusops elengi bark in experimental gastric ulcers. J. Ethnopharmacol.. 2003;89:305-311.
- [Google Scholar]
- Purnima, A., Koti, B.C., Thippeswamy, A.H.M., Jaji, M.S, Viswanatha swamy, A.H.M., Kurhe, Y.V., Jaffar Sadiq, A., 2010. Antiinflammatory, Analgesic and Antipyretic activities of Mimusops elengi Linn. Indian J. Pharm. Sci. 72, 480-485.
- Cytotoxicity activity of extracts and compounds from Commiphora myrrha resin against human gynecologic cancer cells. J. Med. Plants Res.. 2011;5(8):1382-1389.
- [Google Scholar]
- induction of apoptosis and cytotoxic activities of Apium graveolens Linn. Middle-East J. Sci. Res.. 2011;9(1):90-94.
- [Google Scholar]
- Production of triterpenoid anti-cancer compound taraxerol in Agrobacterium- transformed root cultures of butterfly pea (Clitoria ternatea L.) Appl. Biochem. Biotechnol.. 2012;168(3) 487-450
- [Google Scholar]
- Down-regulation of human papillomavirus E6/E7 oncogene by arsenic trioxide in cervical carcinoma cells. Cancer Lett.. 2002;181(1):11-22.
- [Google Scholar]
- Antiproliferative activities of Parthenolide and Golden Feverfew extract against three human cancer cell lines. J. Med. Food.. 2006;9(1):55-61.
- [Google Scholar]







