Translate this page into:
Synthesis, biological screening of novel long chain derivatives of 1,3-disubstituted-1H-pyrazol-5(4H)-one and 2-substituted-3H-1,4-phthalazin-1,4-dione: Structure-activity relationship studies
*Corresponding author. Tel.: +91 9412545345 abduloafchem@gmail.com (Abdul Rauf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 17 September 2013
Peer review under responsibility of King Saud University.

Abstract
The main purpose of this study is to synthesize novel heterocyclic derivatives of fatty acids which are also biologically important. The simple, efficient and one-pot synthesis of two novel series of 1-long chain alkanoyl/alkenoyl/hydroxyalkenoyl-3-methyl-1H-pyrazol-5(4H)-ones 2(a–e) and 2-long chain alkenoyl/hydroxyalkenoyl-3H-phthalazin-1,4-diones 3(b–e) is achieved by the reaction of ethylacetoacetate/phthalic anhydride and long chain alkyl/alkenyl/hydroxyalkenyl hydrazides 1(a–e). Although some methods are available for the synthesis of phthalazindiones and pyrazolones, the development of a new synthetic method for the efficacious build up of heterocycles (phthalazindiones and pyrazolones) substituted with long alkanoyl/alkenoyl/hydroxyalkenoyl chain is an interesting challenge in the field of synthesis of novel compounds of fatty acids that includes heterocyclization and derivatization of fatty acids. Compounds 2(a–e) were synthesized by the cyclization reaction between ethylacetoacetate and long alkyl/alkenyl/hydroxyalkenyl chain hydrazides 1(a–e). Compounds 3(b–e) were synthesized by the reaction of phthalic anhydride and long alkenyl/hydroxyalkenyl chain hydrazides 1(b–e) in absolute ethanol/glacial AcOH. Structures of all the newly synthesized compounds have been elucidated by means of IR, 1H NMR, 13C NMR and MS. Newly synthesized compounds were evaluated for in vitro antibacterial and antifungal activities and their structure–activity relationship studies have been carried out.
Keywords
Pyrazolones
Phthalazindiones
Fatty acids
Biological screening
Structure–activity relationship
1 Introduction
Nowadays, human beings are totally dependent on the medicines derived from heterocyclic rings. In many pharmaceutically active compounds heterocyclic rings are found, because both heterocycles and medicines are interrelated. The phthalazine nucleus also has very marked pharmacological and biological applications due to its antitumour (Loh et al., 2005), antihypertensive (Demirayak et al., 2004), antithrombotic (Johnsen et al., 2003), antidiabetic (Lenz et al., 2002) and antiinflammatory (Dogruer et al., 2004) activities. Pyrazole ring represents an interesting template for medicinal and combinatorial chemistry (Pevarello et al., 2006) because they play a key role in biologically active compounds. Indeed, several pyrazole derivatives have been reported to possess antimicrobial (Vijesha et al., 2011), antiviral (Narule, 2011), antiandrogenic (Amr et al., 2006), analgesic (Parashar et al., 2010), antiinflammatory (Dohutia et al., 2013), antidiabetic (Das et al., 2008) and antioxidant (Mohan and Ananthan, 2011) properties. Pyrazole and pyrazolone derivatives were also reported to possess antiinflammatory, postmenopausal, osteoporosis, antagonists, anticoagulants and angiotensin activities (Lee et al., 2003). In drug molecules such as celecoxib (a) (Singh et al., 2004), pyrazofurine (b) (Dömling and Ugi, 2000) and many others, the pyrazole ring is the key moiety. Literature survey also reveals that (Z)-4-(4-hydroxy-3-methoxy benzylidene)-1-(3-nitrophenyl)-3-phenyl-1H-pyrazol-5(4H)-one (c) (a pyrazolone derivative) was found to be a non-steroidal Farnesoid X Receptor (FXR) selective antagonist (Huang et al., 2012; Ma et al., 2010). Azelastine (d) is a potent, second-generation, selective histamine antagonist (histamine-H1-receptor antagonist) and it is a member of the phthalazinone family, it shows relevant bronchodilatory activity used for treatment of asthma (McTavish and Sorkin, 1989) and has also been reported to induce vasorelaxation in vitro assays (Lee et al., 1990). Commercially available drug, hydralazine (e), is one of the first antihypertensive drugs developed in 1950s and due to its vasodilator action it is considered as lead for introducing new drugs. Hydralazine belongs to phthalazine drug family and directly acts as a smooth muscle relaxant used to treat hypertension by acting as a vasodilator in arteries and arterioles. A slight change in the structure of hydralazine led to the discovery of some phthalazine derivatives which were found to possess antiparasitic, antifungal, antimicrobial, antipsychotic, antineoplastic activities (Olmo et al., 2006). The well known HAV 3C inhibitor (f) is also a phthalazindione derivative which has been found to possess inhibitory properties against hepatitis A virus 3C proteinase (Jain and Vederas, 2004). Pyrazolo[1,2-b]phthalazine-dione derivatives were reported as antiinflammatory, analgesic, antihypoxic and antipyretic agents (Al’-Assar et al., 2002). Our investigations show that the structure of some drugs which are mainly based on pyrazole, pyrazolone, phthalazine and phthalazinone, phthalazindione nuclei (Fig. 1) is closely related to the structure of the target compounds i.e., 1,3-disubstituted-1H-pyrazol-5(4H)-one and 2-substituted-3H-1,4-phthalazindione fatty acid analogues.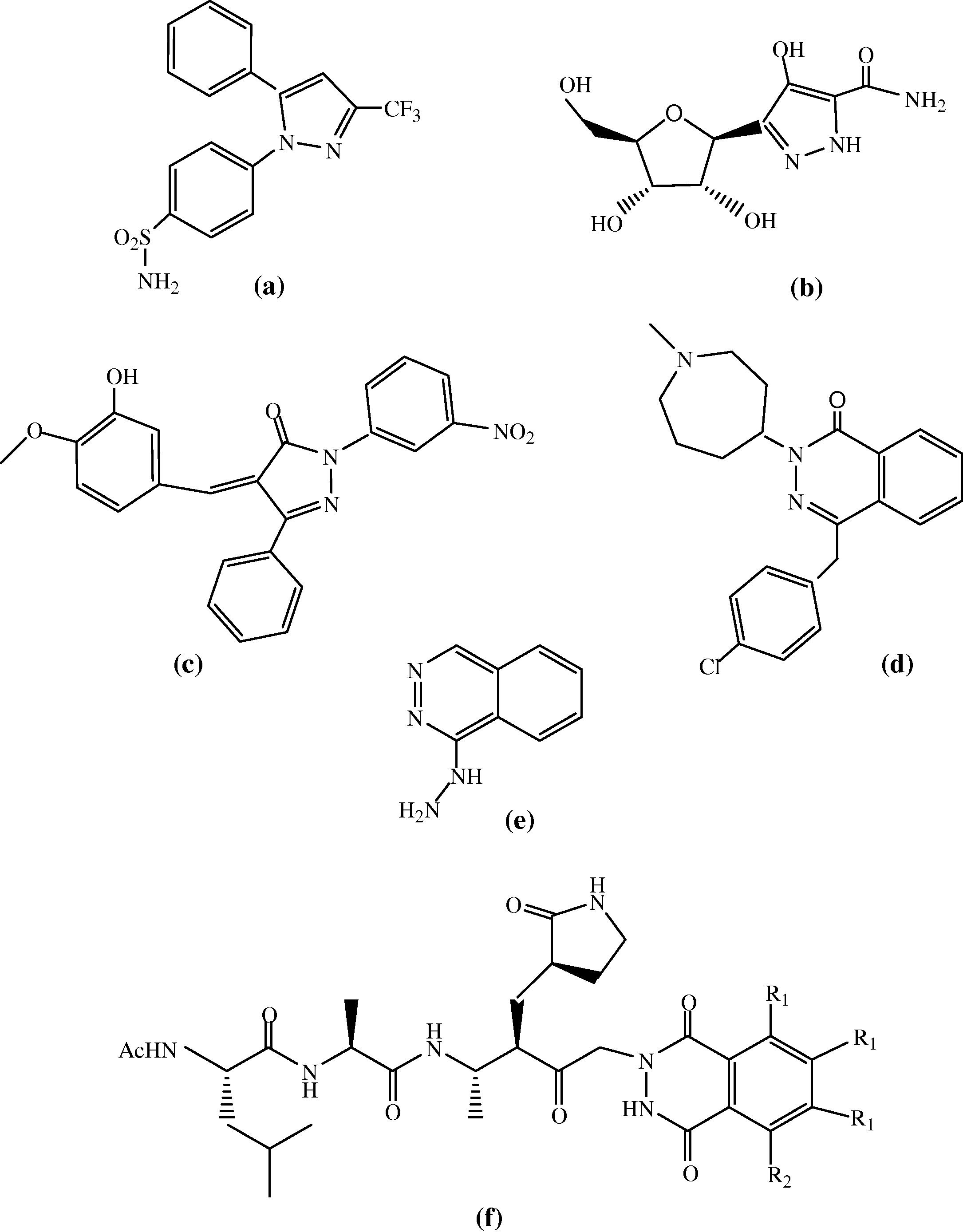
Structurally related drugs to the target compounds.
Carboxylic acids having long alkyl/alkenyl/hydroxyalkenyl chains are found to be pharmacologically active antimicrobial agents (Rauf and Parveen, 2005) and these are also useful in the treatment of renal, cardiovascular and hepatic disorders (Greetings et al., 2003). Also, some fatty acid derivatives have been found to possess antitumor activity and antidepressant activity (Khan et al., 2011; Jubie et al., 2012). Vital utility range of pyrazolones and phthalazindiones, fascinating properties of fatty acids and in continuation of our work in derivatization and heterocyclization of selected fatty acids (Farshori et al., 2010, 2011a,b; Varshney et al., 2013) enthused us to design a simple, efficacious and one-pot synthetic route for the synthesis of two novel series of 1,3-disubstituted-1H-pyrazol-5(4H)-one and 2-substituted-3H-1,4-phthalazindione fatty acid analogues using selected long alkyl/alkenyl/hydroxyalkenyl chain hydrazides as starting materials. Furthermore, this synthetic methodology involves the use of cheap and readily available solvents and commercially available reagents, giving the desired products in good to excellent yields. The ambidexterity of this synthetic route makes it applicable for library synthesis in drug discovery effects.
2 Materials and methods
2.1 Physical and spectroscopic measurements
(9Z, 12R)-12-Hydroxyoctadec-9-enoic and (9R, 12Z)-9-hydroxyoctadec-12-enoic acids were isolated from Ricinus communis and Wrightia tinctoria seed oils respectively following Gunstone’s partition (Gunstone, 1954). Undec-10-enoic acid (Purity 98%), (Z)-octadec-9-enoic acid (97%) and palmatic acid were purchased from Fluka Chemicals (Buck Switzerland). Ethylacetoacetate and phthalic anhydride were purchased from Merck, Mumbai, India. Thin layer chromatography (TLC) was done on glass plated with a layer of silica gel G (Merck, Mumbai, India 0.5 mm thickness). Column chromatography was carried out on silica gel (Merck, Mumbai, India, 60–120 mesh). IR spectra were recorded on Shimadzu 8201 PC spectrometer and absorption given in cm−1. 1H NMR and 13C NMR were recorded in CDCl3 on a Bruker DRX-400 instrument. The chemical shifts (δ) were measured relative to TMS as an internal standard and quoted in ppm. Coupling constants (J) are expressed in Hertz (Hz). The mass spectra were recorded on JEOL-SX 102/DA-600 mass spectrometer.
2.1.1 General procedure for the preparation of 1,3-disubstituted-1H-pyrazol-5(4H)-ones 2(a-e)
Fatty acid hydrazides 1(a–e) previously synthesized in our laboratory (Rauf et al., 2007) were used as the starting material. A mixture of 0.01 mol of fatty acid hydrazides 1(a–e) and 0.1 mol (13 mL) of ethylacetoacetate was heated on paraffin bath. After sometime the reaction mixture turned into a reddish oily syrup. The reaction was continued till all the reactants were consumed. After completion of the reaction, the reaction mixture was cooled and worked up with dichloromethane and water. The product was purified by column chromatography. All the products were oily in nature and were elucidated on the basis of their spectral data.
2.1.2 General procedure for the preparation of 2-substituted-3H-1,4-phthalazinediones 3(b-e)
0.1 mole of fatty acid hydrazides 1(b–e) and 0.1 mol of phthalic anhydride in ethanol (5 mL) were taken in a round bottom flask and 0.005 mol of glacial acetic acid was added. Reaction mixture was refluxed for 10–12 h, and then poured into crushed ice, a solid product was precipitated. All the products were in the form of white powder and were elucidated on the basis of their spectral data. The reaction sequences leading to the formation of these two novel series of compounds are outlined in Scheme 1 and physiochemical parameters of all the newly synthesized compounds are tabulated in the Table 1. ∗M.P.: Melting point.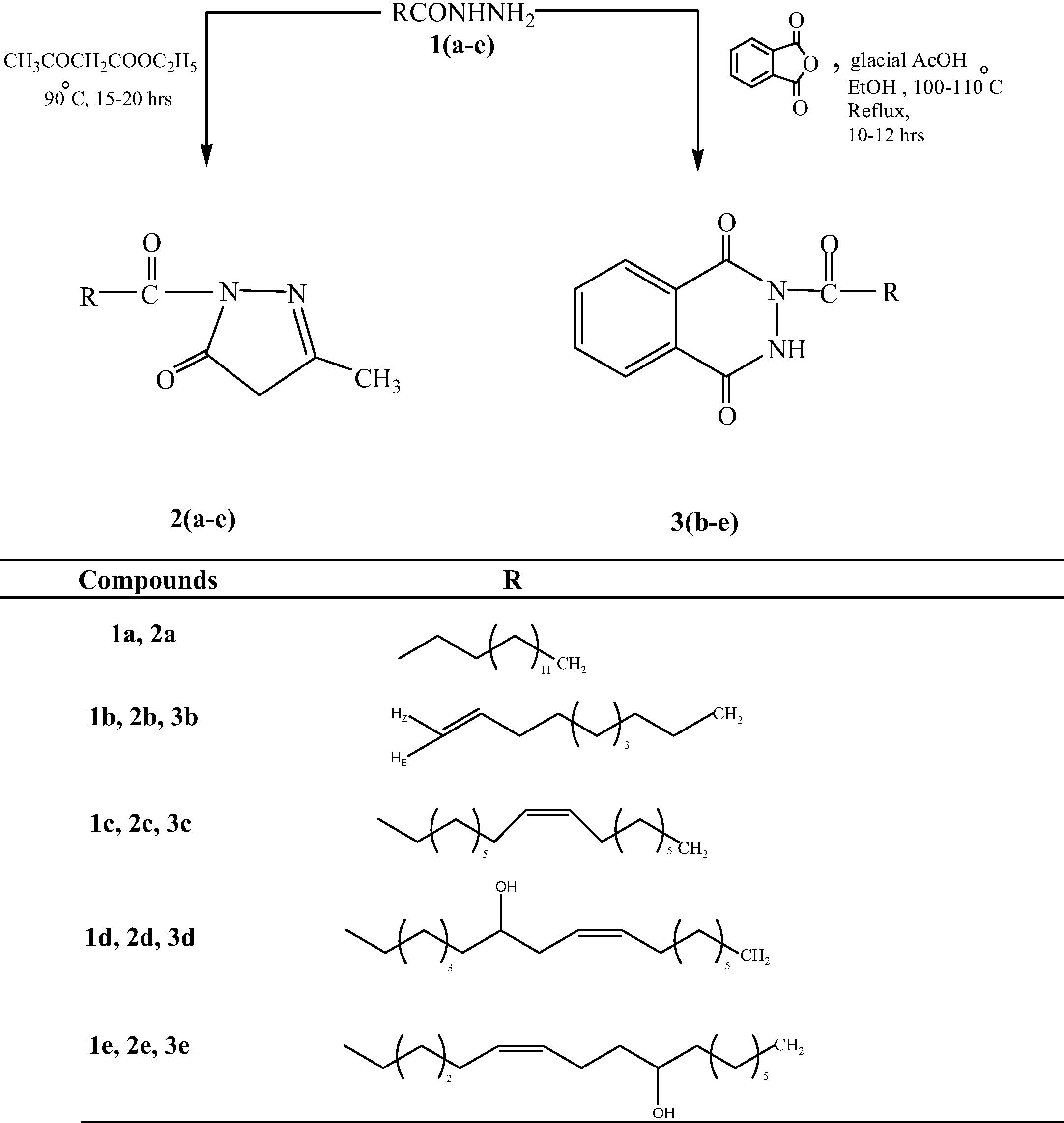
Synthesis of 1,3-disubstituted-1H-pyrazol-5(4H)-ones and 2-substituted-3H-1,4-phthalazindiones.
Code
Compound
Molecular formula
Molecular weight
Physical state
M.P.
% Yield
2a
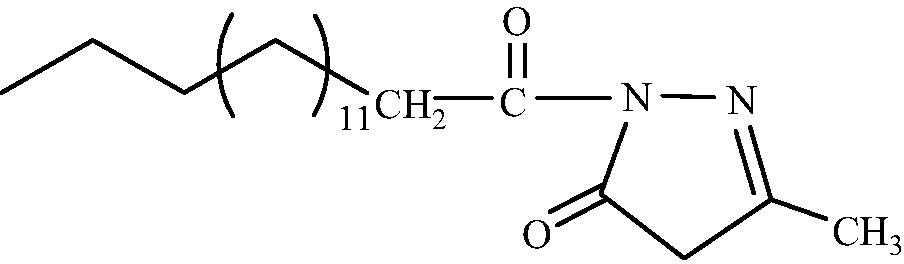
C20H36O2N2
336.44
Colorless oily
–
75
2b
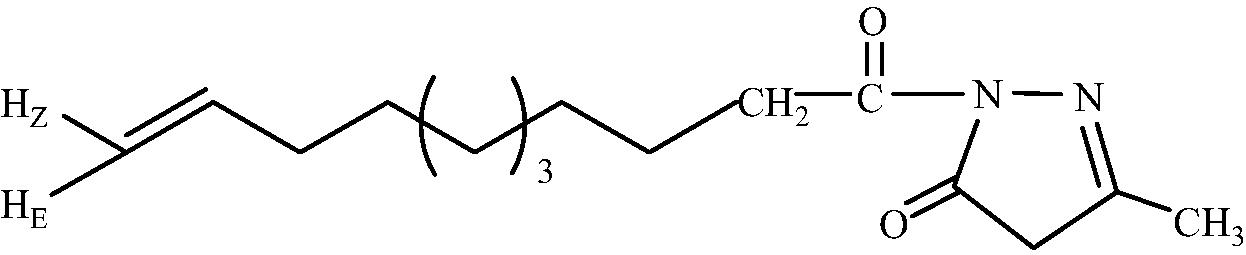
C15H24O2N2
264.32
Colorless oily
–
74
2c
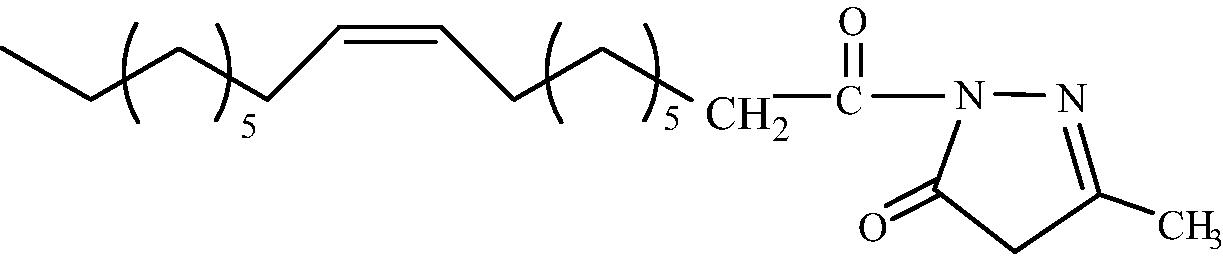
C22H38O2N2
362.48
Reddish brown oily
–
70
2d
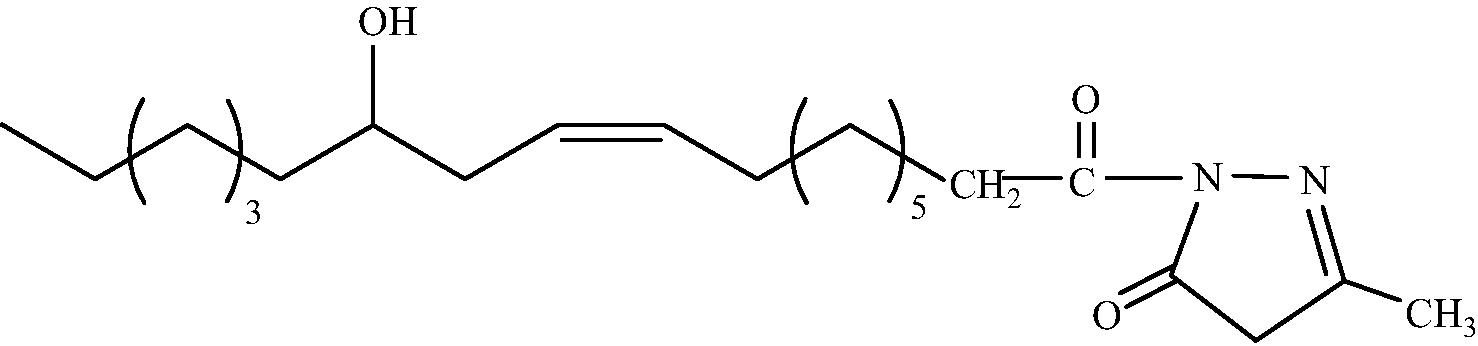
C22H38O3N2
378.48
Reddish brown oily
–
71
2e
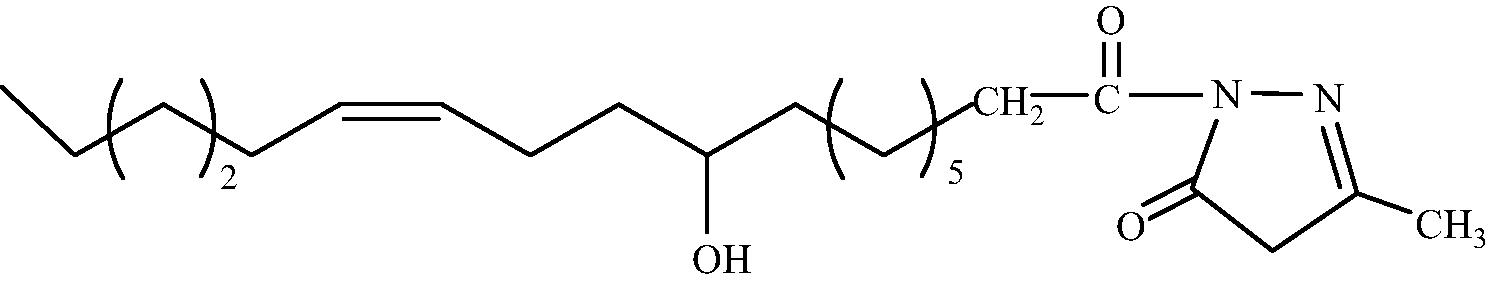
C22H38O3N2
378.48
Reddish brown oily
–
73
3b
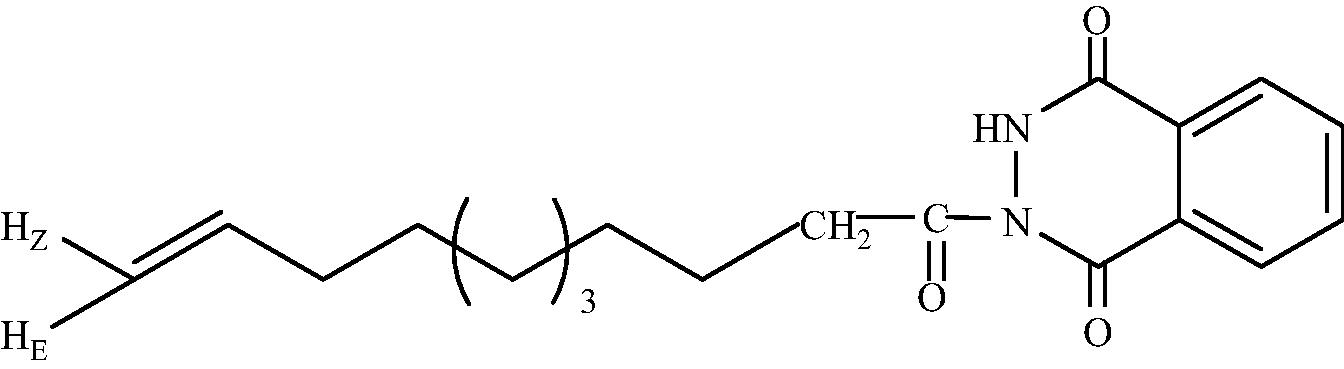
C19H24O3N2
328.36
White powder
85–87
85
3c
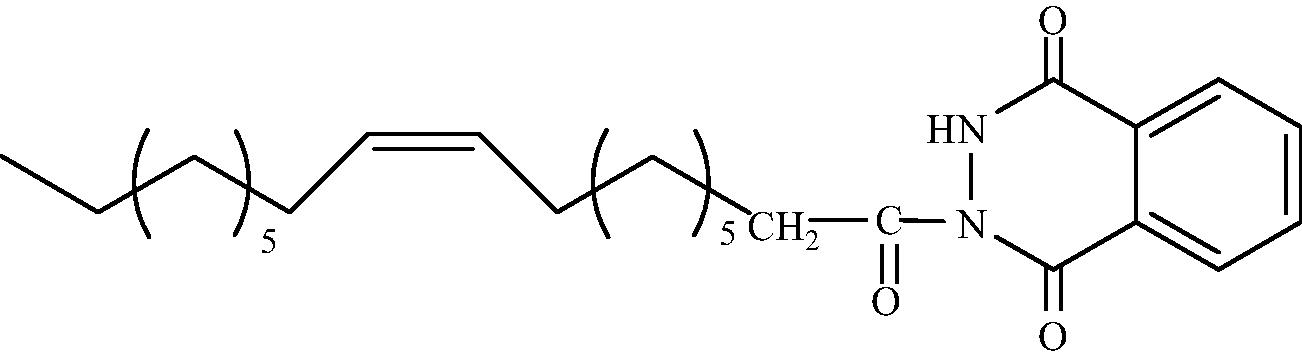
C26H38O3N2
426.52
White powder
91–92
82
3d
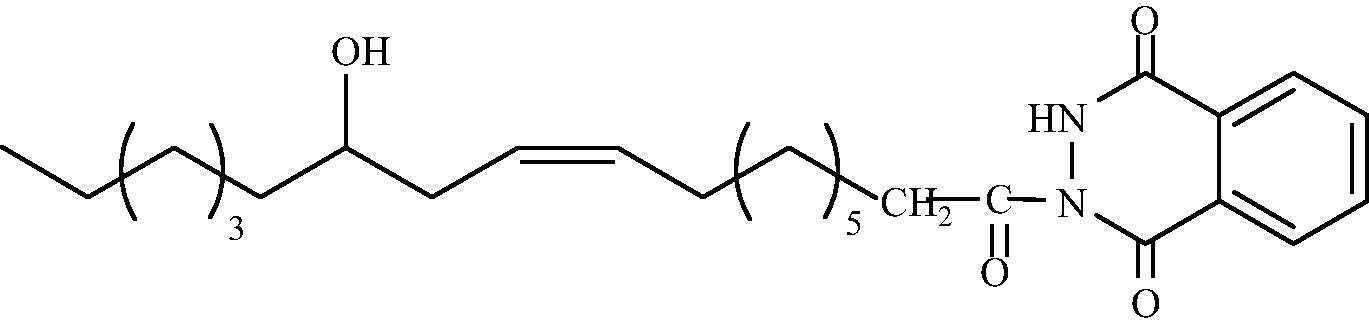
C26H38O4N2
442.52
White powder
70–72
70
3e
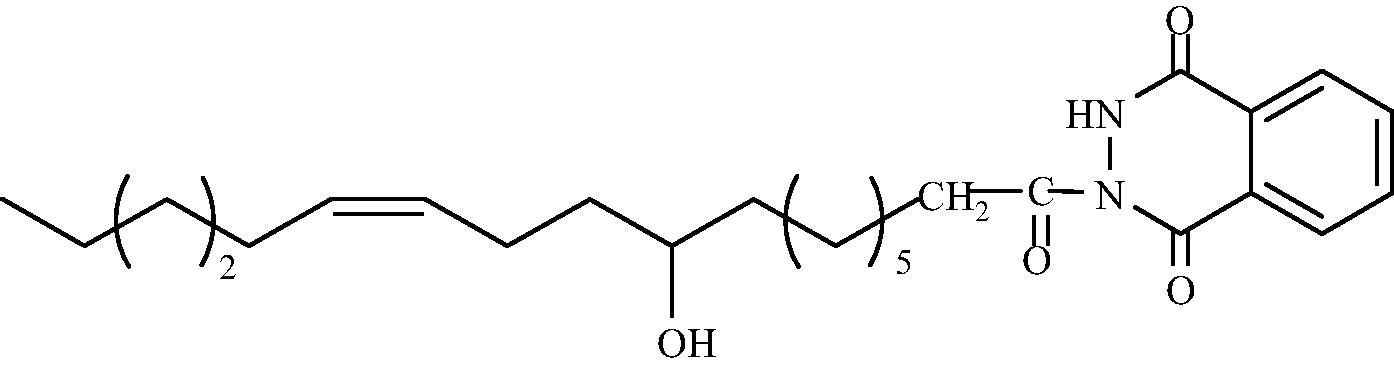
C26H38O4N2
442.52
White powder
74–76
74
2.2 Biological activity
2.2.1 Antibacterial studies
A stock solution of 1 mg/mL was prepared by dissolving all the newly synthesized compounds in DMSO. Filter paper disc method (FPDM) (Bauer et al., 1966) was used for testing the antibacterial activity of all the target compounds and standard doxycycline Media with DMSO was set up as control. On nutrient agar all cultures were systematically maintained and incubated overnight at 37 °C. At 1000 rpm the culture was centrifuged, pellets were resuspended and then diluted in sterile normal saline solution (NSS) to obtain viable 105 cfu/mL. On nutrient agar plates approximately 0.1 mL of diluted bacterial culture suspension was spread uniformly with the help of spreader. Sterile 8 mm discs (Hi-media Pvt. Ltd) were impregnated with the test compounds. Antibiotic disc, doxycycline (30 μg/disc Hi-Media) was used as control. The disc was placed on the nutrient agar plate. Each plate had one control disc impregnated with the solvent. The plates were then incubated for 24 h at 37 °C, and the resulting zones of inhibition were measured (mm).
2.2.2 Antifungal studies
The synthesized compounds were dissolved in DMSO. Media with DMSO was set up as control. All cultures were consistently maintained on SDA (sabouraud’s dextrose agar) and incubated at 28 °C. Spore formation of filamentous fungi was prepared from 7 day old culture in sterile normal saline solution (8% NaCl) and approximately diluted to obtain 105cfu/mL. The inoculums of non-sporing fungi, Candida albicans were performed by growing the culture in SD (sabouraud’s) broth at 37 °C for overnight. At 1000 rpm the culture was centrifuged, pellets were resuspended and then diluted in sterile normal saline solution (NSS) to obtain viable 105 cfu/mL. On SDA plates approximately 0.1 mL of diluted fungal culture suspension was spread uniformly with the help of spreader. Sterile 8 mm discs (Hi-media Pvt. Ltd) were impregnated with the test compounds. Antibiotic disc, nystatin (30 μg/disc Hi-Media) was used as control. The disc was placed onto the SDA plate. Each plate had one control disc impregnated with the solvent. The plates were incubated at 28 °C for filamentous fungi for 72 h or more, while for C. albicans plates were incubated at 37 °C for 18–48 h. Antifungal activity was determined by measuring the diameters of the inhibition zone (mm).
3 Results and discussion
3.1 Chemistry
The present study is based on the synthesis and characterization of two novel series of pyrazolone and phthalazindione derivatives of long chain fatty acids. The fatty acid hydrazides 1(a–e) were used as the starting material and they were prepared from the fatty acids following previously reported method (Rauf et al., 2007). The derivatives of 1,3-disubstituted-1H-pyrazol-5(4H)-ones 2(a–e) were synthesized by the condensation of long chain alkyl, alkenyl (internal and terminal) and hydroxyalkenyl carboxylic acid hydrazides 1(a–e) with ethylacetoacetate, followed by cyclization. The synthesized compounds were identified on the basis of IR, 1H NMR, 13C NMR and MS. The structure of compound 2b was confirmed by the appearance of an absorption band at 1603 cm−1 due to C⚌N stretch. Another absorption band for C⚌O was observed at 1698 cm−1. The absorption band at 2932 cm−1 was observed for aliphatic stretch. The 1H NMR spectrum was more informative in assigning the structure. In addition to peaks of fatty acid chain, the singlet at δ4.18 for two protons of pyrazolone ring CH2 was observed along with a singlet at δ1.57 for CH3 protons which is directly attached to the ring. In 13C NMR, peaks at δ178.6, δ130.2, δ50.8 were observed. Further evidence was obtained by recording mass spectrum. The mass spectrum showed characteristic molecular ion peak which was in accordance with the molecular formula. Similar type of spectral data were obtained for compounds 2a, 2c, 2d and 2e.
Similarly, the other novel series of 2-substituted-3H-1,4-phthalazindione derivatives 3(b–e) were synthesized by the condensation of long chain alkenyl (internal and terminal) and hydroxyalkenyl carboxylic acid hydrazides 1(b–e) with phthalic anhydride in ethanol and catalytic amount of glacial acetic acid was used. Products were in the form of white powder. All the newly synthesized compounds were characterized on the basis of IR, 1H NMR, 13C NMR and MS. Compound 3b showed IR absorption bands at 3228 cm−1 for N-H stretch, at 2925 cm−1 for C-H stretch and at 1599 cm−1 for C⚌O stretch. The 1H NMR showed a singlet at δ8.01 corresponding to the N–H proton. Also a multiplet at δ7.74–7.89 was observed for four aromatic protons. In 13C NMR, peaks at δ171.8, δ165.3, δ139.2, δ134.7, δ130.9, δ129.1, δ128.4, and δ127.1 were observed. The mass spectrum showed a characteristic molecular ion peak which was in accordance with the molecular formula. Similar type of spectral data were obtained for compounds 3c, 3d and 3e.
3.1.1 The spectroscopic and analytical data for the synthesized compounds 2(a–e) and 3(b–e) are presented below
3.1.1.1 1-(Hexadecanoyl)-3-methyl-1H-pyrazol-5(4H)-one (2a)
IR (KBr, cm−1): 2920 (C–H stretching), 1680 (C⚌O stretching), 1592 (C⚌N stretching). 1H NMR (CDCl3, δH): 4.12 (2H, s, CH2 ring), 2.25 (2H, t, J = 7.59 Hz, CH2CO), 1.95 (2H, m, CH2CH2CO), 1.56 (3H, s, CH3 ring), 1.22 (24H, br.s, (CH2)12 chain), 0.85 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 176.9, 165.4, 127.9, 45.8, 35.6, 33.8, 33.7, 32.9, 31.8, 31.2, 30.0, 29.9, 29.8, 29.7, 27.4, 27.0, 25.4, 23.8, 22.8, 14.0. MS (ESI): m/z = 359.500 found [M + Na]+, calculated [M + Na]+ = 401.470.
3.1.1.2 1-(Undec-10′-enoyl)-3-methyl-1H-pyrazol-5(4H)-one (2b)
IR (KBr, cm−1): 2932 (C-H stretching), 1698 (C⚌O stretching), 1603 (C⚌N stretching). 1H NMR (CDCl3, δH): 5.82 (1H, tdd, , , =17.10 HZ, CH2 = CH), 5.01 (1H, dd, , , HZC = CH), 4.91 (1H, dd, , , HEC = CH), 4.18 (2H, s, CH2 ring), 2.51 (2H, t, J = 7.38 Hz, CH2CO), 2.06 (2H, m, CH2 = CH-CH2), 1.71 (2H, m, CH2CH2CO), 1.57 (3H, s, CH3), 1.25 (10H, br.s, CH2(CH2)5). 13C NMR (CDCl3, δC): 178.6, 174.0, 130.2, 130.0, 129.9, 50.8, 34.4, 33.9, 31.9, 30.8, 30.1, 29.7, 27.1, 24.2, 18.0. MS (ESI): m/z = 287.121 found [M + Na]+, calculated [M + Na]+ = 287.310.
3.1.1.3 1-[(9′Z) (Octadec-9′-enoyl)]-3-methyl-1H-pyrazol-5(4H)-one (2c)
IR (KBr, cm−1): 2924 (C-H stretching), 1700 stretching, 1598 (C = N stretching). 1H NMR (CDCl3, δH): 5.35 (2H, m, CH = CH), 4.20 (2H, s, CH2 ring), 2.36 (2H, t, J = 7.52 HZ, CH2CO), 2.24 (4H, m, CH2CH = CHCH2), 2.00 (2H, m, CH2CH2CO), 1.61 (3H, s, CH3), 1.25 (20H, br.s, (CH2)10), 0.88 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 180.1, 165.8, 127.2, 123.1, 122.9, 48.1, 35.0, 33.1, 31.9, 29.7, 29.6, 29.4, 29.3, 29.2, 29.1, 27.1, 25.3, 25.0, 24.7, 15.4, 14.1. MS (ESI) m/z = 385.381 found [M + Na]+, calculated [M + Na]+ = 385.471.
3.1.1.4 1-[(9′Z, 12′R)-12′-Hydroxy-octadec-9′-enoyl]-3-methyl-1H-pyrazol-5(4H)-one (2d)
IR (KBr, cm−1): 3400 (O-H stretching), 2929 (C–H stretching), 1685 (C = O stretching), 1579 (C = N stretching). 1H NMR (CDCl3, δH): 5.34 (2H, m, CH = CH), 4.87 (1H, m, CHOH), 4.11 (2H, s, CH2 ring), 2.27 (2H, t, J = 7.47 HZ, CH2CO), 2.21 (1H, m, CHOH), 1.96 (4H, m, CH2CH = CHCH2), 1.85 (2H, m, CH2CH2CO), 1.55 (3H, s, CH3 ring), 1.21 (18H, br.s, (CH2)9), 0.80 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 182.9, 173.9, 133.2, 132.8, 123.8, 72.0, 50.1, 37.4, 36.7, 34.3, 31.9, 31.7, 30.1, 29.6, 29.5, 29.1, 28.9, 27.3, 25.6, 24.9, 15.9, 14.1. MS (ESI) m/z = 401.299 found [M + Na]+, calculated [M + Na]+ = 401.470.
3.1.1.5 1-[(9′R, 12′Z)-9′-Hydroxy-octadec-12′enoyl]-3-methyl-1H-pyrazol-5(4H)-one (2e)
IR (KBr, cm−1): 3391 (O-H stretching), 2933 (C–H stretching), 1680 (C = O stretching), 1570 (C = N stretching). 1H NMR (CDCl3, δH): 5.35 (2H, m, CH = CH), 4.86 (1H, m, CHOH), 4.13 (2H, s, CH2 ring), 2.25 (2H, t, J = 7.50 HZ, CH2CO), 2.19 (1H, m, CHOH), 1.95 (4H, m, CH2CH = CHCH2), 1.86 (2H, m, CH2CH2CO), 1.53 (3H, s, CH3 ring), 1.23 (18H, br.s, (CH2)9), 0.82 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 181.9, 176.9, 133.1, 132.5, 123.0, 72.9, 50.4, 38.4, 37.7, 33.3, 31.8, 31.7, 30.2, 29.5, 29.2, 29.0, 28.6, 27.1, 25.7, 24.6, 15.9, 14.3. MS (ESI) m/z = 401.302 found [M + Na]+, calculated [M + Na]+ = 401.470.
3.1.1.6 2-(Undec-10′-enoyl)-3H-1,4-phthalazindione (3b)
IR (KBr, cm−1): 3228 (N-H stretching), 2925 (C–H stretching), 1599 (C = O stretching). 1H NMR (CDCl3, δH): 8.01 (1H, s, NH), 7.74–7.89 (4H, m, ArH), 5.80 (1H, tdd, =6.71 HZ, =10.90 HZ, =16.82 HZ, CH2 = CH), 5.01 (1H, dd, =10.11 HZ, =2.20 HZ, HZC = CH), 4.93 (1H, dd, =17.45 HZ, =2.20 HZ, HEC = CH), 2.39 (2H, t, J = 7.50 Hz, CH2CO), 2.03 (2H, m, CH2 = CH–CH2), 1.70 (2H, m, CH2CH2CO), 1.28 (10H, br.s, CH2(CH2)5). 13C NMR (CDCl3, δC): 175.1, 171.8, 165.3, 139.2, 134.7, 130.9, 129.1, 128.4, 127.1, 124.0, 114.1, 33.9, 33.8, 29.3, 29.2, 29.0, 28.9, “one signal hidden”, 25.1. MS (ESI): m/z = 351.333 found [M + Na]+, calculated [M + Na]+ = 351.353.
3.1.1.7 2-[(Z)-Octadec-9′-enoyl]-3H-1,4-phthalazidione (3c)
IR (KBr, cm−1): 3232 (N-H stretching), 2922 (C–H stretching), 1594 (C = O stretching). 1H NMR (CDCl3, δH): 8.36 (1H, s, NH), 7.77–7.92 (4H, m, ArH), 5.34 (2H, m, CH = CH), 2.41 (2H, t, J = 7.40 HZ, CH2CO), 2.22 (4H, m, CH2CH = CHCH2), 1.70 (2H, m, CH2CH2CO), 1.29 (20H, br.s, (CH2)10), 0.87 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 174.8, 170.1, 167.2, 138.1, 134.9, 133.5, 131.9, 128.1, 127.7, 118.4, 115.6, 36.6, 34.2, 31.5, 31.2, 30.5, “two signals hidden”, 29.5, 29.2, 29.0, 28.7, 27.5, 26.2, 24.1, 14.1. MS (ESI) m/z = 449.500 found [M + Na]+, calculated [M + Na]+ = 449.510.
3.1.1.8 2-[(9′Z, 12′R)-12′-Hydroxy octadec-9′-enoyl]-3H-1,4-phthlazindione (3d)
3392 (O-H stretching), 3227 (N-H stretching), 2921 (C–H stretching), 1585 (C = O stretching). 1H NMR (CDCl3, δH): 8.41 (1H, s, NH), 7.34–7.67 (4H, m, ArH), 5.50 (2H, m, CH = CH), 4.21 (1H, m, CHOH), 2.54 (2H, t, J = 7.79 HZ, CH2CO), 2.19 (1H, m, CHOH), 1.80 (4H, m, CH2CH = CHCH2), 1.65 (2H, m, CH2CH2CO), 1.27 (18H, br.s, (CH2)9), 0.88 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 173.1, 168.8, 163.9, 134.9, 133.4, 132.7, 130.1, 126.7, 123.4, 118.8, 114.7, 72.0, 38.1, 38.0, 37.2, 36.7, 35.1, 33.9, “one signal hidden”, 32.1, 29.4, 28.5, 27.6, 24.8, 23.1, 13.9. MS (ESI) m/z = 465.490 found [M + Na]+, calculated [M + Na]+ = 465.513.
3.1.1.9 2-[(9′R, 12′Z)-9′-Hydroxy octadec-12′-enoyl]-3H-1,4-phthalazindione (3e)
3372 (O–H stretching), 3217 (N–H stretching), 2918 (C–H stretching), 1591 (C = O stretching). 1H NMR (CDCl3, δH): 8.42 (1H, s, NH), 7.29–7.57 (4H, m, ArH), 5.480 (2H, m, CH = CH), 4.29 (1H, m, CHOH), 2.34 (2H, t, J = 7.59 HZ, CH2CO), 2.20 (1H, m, CHOH), 1.81 (4H, m, CH2CH = CHCH2), 1.71 (2H, m, CH2CH2CO), 1.25 (18H, br.s, (CH2)9), 0.81 (3H, dist.t, CH3). 13C NMR (CDCl3, δC): 174.5, 169.1, 162.6, 134.0, 133.9, 132.5, 131.5, 125.7, 124.4, 119.0, 112.9, 71.0, 38.9, 38.1, 37.6, 36.7, 35.8, 33.0, “one signal hidden”, 32.1, 30.0, 29.5, 28.4, 26.4, 24.9, 14.4. MS (ESI) m/z = 465.600 found [M + Na]+, calculated [M + Na]+ = 465.513.
3.2 Biology
All the newly synthesized compounds were assessed for in vitro antibacterial activity against an assortment of two Gram-positive bacteria, Staphylococcus aureus SA 22, Bacillus subtilis MTCC 121, and two Gram-negative bacteria, Escherichia coli K12, Pseudomonas aeruginosa. Doxycycline was used as the standard drug for the comparison of the antibacterial activity results. The in vitro antimicrobial screening results are given in Table 2. Graphic representation of the biological screening results is depicted in Fig. 2(A–E) in terms of diameter of zone inhibition (in mm). The newly synthesized compounds 2(a–e) and 3(b–e) have exerted significant inhibitory activity against the growth of the tested bacterial strains. The antibacterial screening results showed that among the tested bacterial strains, good inhibitory results were obtained against E. coli and P. aeruginosa as depicted in Fig. 2(C) and (D). Among the tested compounds, 3d and 3e showed more potent inhibitory activity against both types of bacteria. However, depending on the nature of the heterocyclic moiety (phthalazindione/pyrazolone) and substituents (long alkanoyl/alkenoyl/hydroxy alkenoyl chains) attached to it exerted varying inhibitory actions. In another set of experiments, all the newly synthesized compounds 2(a–e) and 3(b–e) were also screened for in vitro antifungal activity against C. albicans IOA 109. Nystatin was used as the standard drug for the comparison of the antifungal results. The synthesized compounds showed moderate to excellent inhibitory results for C. albicans as seen in Fig. 2(E). ∗NA: Not applicable.
Test sample
Concentration (μg/mL)
Bacillus subtilis
Staphylococcus aureus
Escherichia coli
Pseudomonas aeruginosa
Candida albicans
2a
400
–
–
10
11
–
800
–
–
14
17
13
2b
400
–
–
14
16
11
800
15
14
22
19
16
2c
400
–
–
13
13
10
800
–
–
17
19
14
2d
400
11
10
15
15
14
800
13
13
22
21
18
2e
400
10
11
13
13
13
800
13
14
19
20
17
3b
400
–
–
14
13
11
800
11
13
20
16
17
3c
400
–
–
14
–
10
800
–
–
19
14
15
3d
400
13
11
18
14
13
800
17
15
22
24
19
3e
400
11
11
15
15
14
800
16
15
23
22
20
Doxycycline
30 (μg/disc)
20
18
23
21
NA
Nystatin
30 (μg/disc)
NA
NA
NA
NA
18
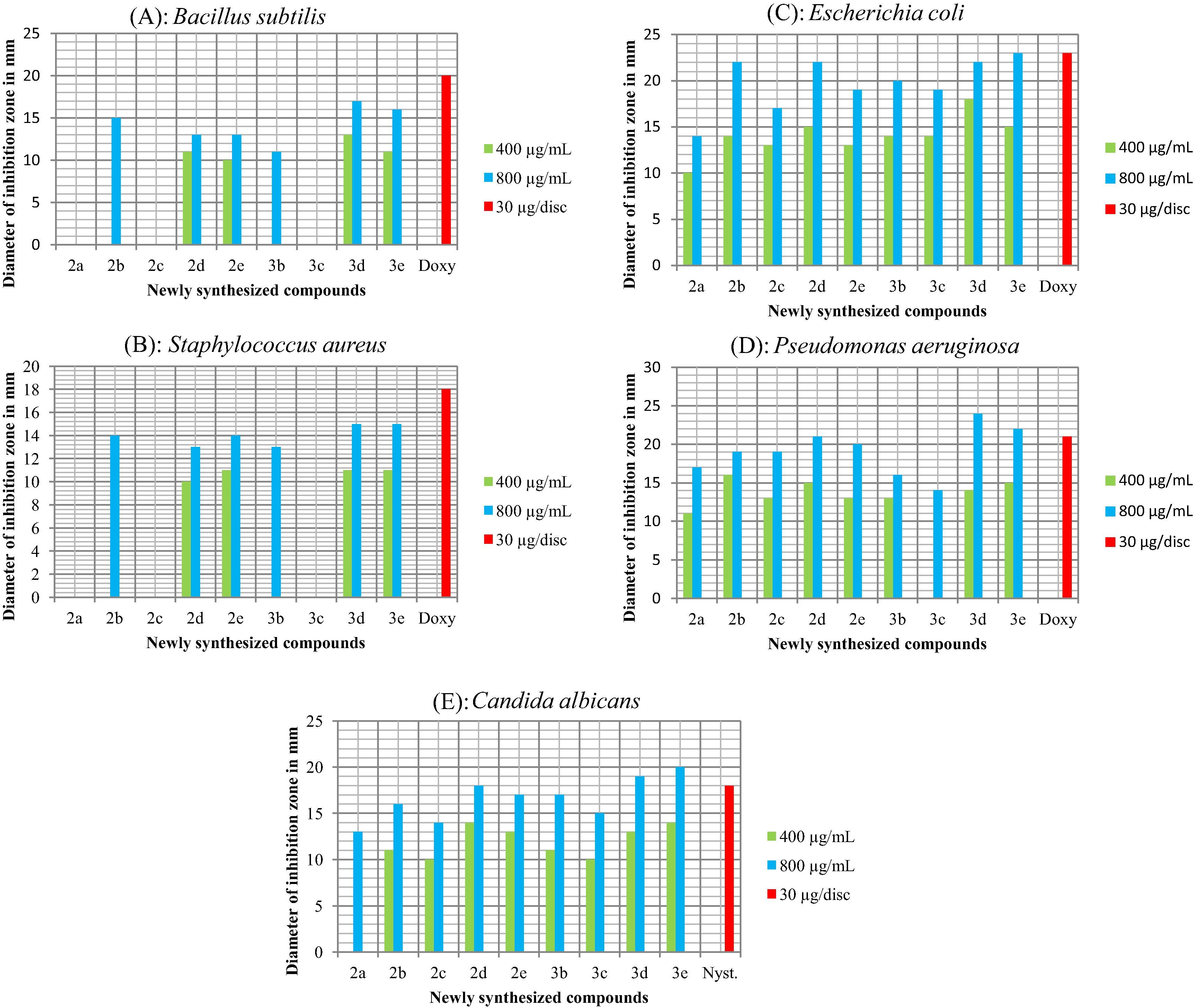
(A–E): Graphic representation of the antimicrobial screening results in terms of diameter of inhibition zone (in mm) against various microbial strains. Fig. 2(A–D) shows the antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, respectively, standard used was Doxycycline. Fig. 2(E) shows the antifungal activity against Candida albicans, standard used was Nystatin.
3.2.1 Structure–activity relationship (SAR) studies
The structure–activity studies showed that depending on the nature of the heterocyclic skeleton and its substituent, the newly synthesized compounds 2(a–e) and 3(b–e) exhibit varying degree of microbial inhibition. From the antimicrobial screening results, it has been found that the phthalazindione derivatives 3(b–e) have shown better activity as compared to their corresponding pyrazolone derivatives 2(a–e). Within the same heterocyclic series that is either in phthalazindione derivatives or in pyrazolone derivatives, the inhibitory activity depends on the nature of substituents attached to them. The in vitro biological screening indicated that the presence of the hydroxy substituents attached to the heterocyclic moiety as in compounds (2d, 2e, 3d and 3e) increased their inhibitory activities compared to other compounds. Structure activity relationship (SAR) studies cleared that, compounds with terminal alkenoyl substituent (2b and 3b) and smaller carbon chain (C10) have better inhibitory activity compared with compounds (2c and 3c) which have internal alkenoyl substituent and longer carbon chain (C17). Further, SAR studies show that heterocyclic moiety having alkanoyl substituent (2a) which is analogue of saturated fatty acid and having long carbon atom chain (C15) shows very small inhibitory results. The values of diameter of zone of inhibition in mm for all the newly synthesized compounds are pictorially represented in graphical form in Fig. 2(A–E) against B. subtilis, S. aureus, E. coli, Pseudomonas aeruginosa and C. albicans, respectively. The maximum inhibition was observed in phthalazindione derivatives substituted with hydroxy substituent i.e., 3d and 3e against P. aeruginosa with zone diameter of 14 and 15 mm, respectively (at 400 μg/mL concentration) and also with zone diameter of 24 and 22 mm, respectively (at 800 μg/mL concentration) as seen in Fig. 2(D). The compounds 3d and 3e also show good inhibitory activity against E. coli with zone diameter of 18 and 15 mm, respectively (at 400 μg/mL concentration) and with zone diameter of 22 and 23 mm, respectively (at 800 μg/mL concentartion) as depicted in Fig. 2(C). Hence, we summarized that higher activity of compounds 2d, 2e, 3d and 3e may be attributed to the presence of hydroxy substituents. Also, the synthesized compounds with phthalazindione rings substituted with hydroxy group (3d and 3e) show excellent activity as compared to pyrazolone rings substituted with hydroxy group (2d and 2e). Thus, the nature of substituents and the heterocyclic skeleton of molecules have a strong influence on the extent of antibacterial and antifungal activities.
4 Conclusion
To the best of our knowledge these alkanoyl/alkenoyl/hydroxyalkenoyl pyrazolone and phthalazindione derivatives of some selected fatty acids have been synthesized for the first time. The salient features of these procedures includes mild reaction conditions, use of inexpensive reagents and starting material, products were obtained in good to excellent yields. The structure–activity studies showed that depending on the nature of heterocyclic skeleton and its substituent, the newly synthesized compounds 2(a–e) and 3(b–e) exhibit varying degree of microbial inhibition. The in vitro biological screening results show that compounds having hydroxy substituent attached to the heterocyclic moiety (2d, 2e, 3d and 3e) were the most promising antimicrobial agents. The maximum inhibition was observed in phthalazindione derivatives substituted with hydroxy substituent i.e., 3d and 3e. The synthesis will be a valuable addition to the synthetic methodology available for the synthesis of heterocyclic derivatives of fatty acids which are useful biologically as well as industrially. From these studies it is understandable that further derivatization and heterocyclization of these hetero-analogues of fatty acids can be served as new templates for antimicrobial drug discovery and could probably lead to more potent agents in this field.
Acknowledgments
Authors thank to the Chairman, D/O Chemistry, AMU, Aligarh for providing the necessary research facilities and the Director, SAIF, Punjab University, Chandigarh for recording the spectra. Two of us, A.A. and H.V. are also thankful to CSIR and DST, New Delhi, for the award of Junior Research Fellowship. In part, research is also supported by UGC-SAP(DRS-I) funds.
References
- Synthesis and pharmacological activity of 1-hydroxy-, 1-amino-, and 1-hydrazino- substituted 2,3-dihydro-1H-pyrazolo[1,2-A]pyridazine-5,8-diones and 2,3-dihydro-1H- pyrazolo[1,2-b]phthalazine-5,10-diones. Pharm. Chem. J.. 2002;36:598-603.
- [Google Scholar]
- Synthesis and antiandrogenic activity of some new 3-substituted androstano[17,16-c]-5’-aryl-pyrazoline and their derivatives. Bioorg. Med. Chem.. 2006;14:373-384.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45:493-496.
- [Google Scholar]
- Synthesis and biological evaluation of some new aryl pyrazol-3-one derivatives as potential hypoglycemic agents. Indian J. Chem.. 2008;47B:1555-1558.
- [Google Scholar]
- Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem.. 2004;39:1089-1095.
- [Google Scholar]
- Synthesis of new 2-[1(2H) phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and antiinflammatory agents. Arch. Pharmacol.. 2004;337:303-310.
- [Google Scholar]
- Synthesis and study of analgesic, anti inflammatory activities of 3-methyl-5-pyrazolone derivatives. Int. J. Pharm. Pharm. Sci.. 2013;5:86-90.
- [Google Scholar]
- Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed.. 2000;39:3168-3210.
- [Google Scholar]
- Synthesis, characterization, and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2- phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg. Med. Chem. Lett.. 2010;20:1933-1938.
- [Google Scholar]
- A facile, one-pot synthesis, characterization and antimicrobial activity of o-hydroxy anilide derivatives and 1- substituted-1,3-dicyclohexylurea analogs of long chain carboxylic acids. Eur. J. Med. Chem.. 2011;46:1433-1438.
- [Google Scholar]
- 7-Hydroxy- coumarin derivatives: Synthesis, characterization and preliminary antimicrobial activities. Med. Chem. Res.. 2011;20:535-541.
- [Google Scholar]
- Fatty Acids, Part-II. The nature of the oxygenated acid present in Vernonia anthelmintica (Wild.) seed oil. J. Chem. Soc. 1954:1611-1616.
- [Google Scholar]
- Discovery and optimization of 1,3,4- trisubstituted-pyrazolone derivatives as novel, potent and non-steroidal Farnesoid X Receptor (FXR) selective antagonists. J. Med. Chem.. 2012;55:7037-7053.
- [Google Scholar]
- Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase. Bioorg. Med. Chem. Lett.. 2004;14:3655-3658.
- [Google Scholar]
- New antithrombotic 1- phthalazinamines with serotonin antagonistic properties. Arch. Pharmacol.. 2003;336:591-597.
- [Google Scholar]
- Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur. J. Med. Chem.. 2012;54:931-935.
- [Google Scholar]
- Synthesis and characterization of novel PUFA esters exhibiting potential anticancer activities: An in vitro study. Eur. J. Med. Chem.. 2011;46:4878-4886.
- [Google Scholar]
- Effects of azelastine on contraction of guinea pig tracheal smooth muscle. Eur. J. Pharm.. 1990;187:67-74.
- [Google Scholar]
- Regioselective synthesis of 1,3,4,5-tetrasubstituted pyrazoles from Baylis–Hillman adducts. Tetrahedron Lett.. 2003;44:6737-6740.
- [Google Scholar]
- A comparison of quantitative NMR and radiolabelling studies of the metabolism and excretion of Statil (3-(4-bromo-2-fluorobenzyl)-4-oxo-3H- phthalazin-1-ylacetic acid) in the rat. J. Pharm. Biomed. Anal.. 2002;28:31-43.
- [Google Scholar]
- Phthalazinones. Part 1: The design and synthesis of a novel series of potent inhibitors of poly (ADP- ribose) polymerase. Bioorg. Med. Chem. Lett.. 2005;15:2235-2238.
- [Google Scholar]
- Microwave-assisted one- pot synthesis of pyrazolone derivatives under solvent-free conditions. Molecules. 2010;15:3593-3601.
- [Google Scholar]
- Azelastine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1989;38:778-800.
- [Google Scholar]
- A new approach for the synthesis of some novel sulphur bridged pyrazoles and their characterization. J. Chem. Pharm. Res.. 2011;3:402-413.
- [Google Scholar]
- Synthesis of some new 1-N- (β -D-glucopyranosyl)-2-((1-phenyl-5- aryl)-pyrazol-3-yl) pyrroles and their biological activities. J. Chem. Pharm. Res.. 2011;3:38-47.
- [Google Scholar]
- Vasorelaxant activity of phthalazinones and related compounds. Bioorg. Med. Chem. Lett.. 2006;16:2786-2790.
- [Google Scholar]
- Comparative Conventional and Microwave assisted synthesis of some pyrazoline derivatives and their antimicrobial activity. J. Chem. Pharm. Res.. 2010;2:33-42.
- [Google Scholar]
- 3-Amino-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazoles: a new class of CDK2 inhibitors. Bioorg. Med. Chem. Lett.. 2006;16:1084-1090.
- [Google Scholar]
- Preparation, characterization and antimicrobial activity of fatty alkenoates. Ind. J. Chem.. 2005;44B:1273-1276.
- [Google Scholar]
- Microwave assisted efficient one-pot synthesis of 3,5,6-trisubstituted-1,2,4-triazines from fatty acid hydrazides under solvent-free conditions and their antimicrobial activity. ARKIVOC xvi 2007:137-147.
- [Google Scholar]
- Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors. Bioorg. Med. Chem. Lett.. 2004;14:499-504.
- [Google Scholar]
- Synthesis and evaluation of in vitro antimicrobial activity of novel 2,3-disubstituted-4- thiazolidinones from fatty acid hydrazides. Med. Chem. Res.. 2013;22:3204-3212.
- [Google Scholar]
- Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Der Pharma Chemica. 2011;3:454-463.
- [Google Scholar]







