Translate this page into:
Synthetic biolubricant basestocks based on environmentally friendly raw materials
*Corresponding author. Tel.: +60 3 8921 4574; fax: +60 3 8921 5410 nadiaas@ukm.my (Nadia Salih)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 24 February 2011
Abstract
This work outlines modifications in the epoxidation, oxirane ring opening, estrification and acylation reactions to produce oleic acid based triester derivatives. Measuring of pour point (PP), flash point (FP), viscosity index (VI), oxidation onset temperature (OT) and signal maximum temperature (SMT) was carried out for each compound. The resulting product structures were confirmed by NMR and FTIR spectroscopic analysis. The results showed that butyl 9-(decanoyloxy)-10-(behenoxy)octadecanoate with bulky ester behenyl mid chain exhibited the most favorable low-temperature performance (PP −47 °C). On the other hand, butyl 9-(decanoyloxy)-10-(octyloxy)octadecanoate exhibited higher oxidation stability (OT 173 °C) than the other synthetic esters. In conclusion, an increase in mid chain substituent length improves the PP and conversely lowers the OT.
Keywords
Synthetic biolubricants
Acylation reaction
Pour point
Oxidative stability
1 Introduction
Synthetic biolubricants are used for two major reasons: when an equipment demands specific performance features that cannot be met with conventional mineral oil-based lubricants, examples are extreme high or low operating temperature, stability under extreme conditions and long service life and when synthetic biolubricants can offer economic benefits for overall operation, such as reduced energy consumption, reduced maintenance and increased power output (Bartz, 2006).
Conventional lubricants are formulated based on mineral oils derived from petroleum. Mineral oil contains many classes of chemical components, including paraffins, naphthenes, aromatics, hetero-atom species, etc. Its compositions are pre-determined by the crude source. Modern oil refining processes remove and/or modify the molecular structures to improve the lubricant properties, but are limited in their ability to substantially alter the initial oil composition to fully optimize the hydrocarbon structures and composition (Kodali and Nivens, 2001). Mineral oils of such complex compositions are good for general-purpose lubrication, but are not optimized for any specific performance feature. The major advantages for mineral oils are their low cost, long history and user’s familiarity. But this paradigm is now changing (Puşcaş et al., 2006).
The trend with modern machines equipment is to operate under increasingly more severe conditions, to last longer, to require less maintenance and to improve energy efficiency. In order to maximize machine performance, there is a need for optimized and higher performance lubricants. Synthetic biolubricants are designed to maximize lubricant performance to match the high demands of modern machines equipment and to offer tangible performance and economic benefits (Schuchart et al., 1998).
Synthetic biolubricants differ from conventional lubricants in the type of components used in the formulation. The major component in a synthetic biolubricant is the synthetic base stock. Synthetic base stocks are produced from carefully-chosen and well-defined chemical compounds and by specific chemical reactions. The final base stocks are designed to have optimized properties and significantly improved performance features meeting specific equipment demands (Guzman, 2002). The most commonly optimized properties are
Viscosity index (VI). VI is a number used to gauge oil’s viscosity change as a function of temperature. Higher VI indicates less viscosity change as oil temperature changes – a more desirable property.
Pour point and low temperature properties. Many synthetic base stocks have low PP, −30 to −70 °C, and superior low-temperature properties. Combination of low pour point and superior low-temperature properties ensures oil flow to critical engine parts during cold starting, thus, offering better lubrication and protection. Conventional mineral oils typically have pour points in the range of 0 to −20 °C. Below these temperatures, wax crystallization and oil gelation can occur, which prevent the flow of lubricant to critical machine parts.
Thermal/oxidative stability. When oil oxidation occurs during service, oil viscosity and acid content increase dramatically, possibly corroding metal parts, generating sludge and reducing efficiency. These changes can also exacerbate wear by preventing adequate oil flow to critical parts. Although oil oxidation can be controlled by adding antioxidants, in the long term service and after the depletion of antioxidant, the intrinsic oxidative stability of a base stock is an important factor in preventing oil degradation and ensuring proper lubrication (Fox and Stachowiak, 2007). Many synthetic base stocks are designed to have improved thermal oxidative stability, to respond well to antioxidants and to resist aging processes better than mineral oil.
The aim of this study was to present a novel synthetic approach for the chemical modification of oleic acid to yield oleic acid-based triester derivatives with improved physicochemical properties.
2 Experimental
2.1 Materials
Formic acid (88%) was obtained from Fisher Scientific (Pittsburgh, PA) and oleic acid (99%) from Nu-Chek Prep, Inc. (Elysian, MN). All other chemicals and reagents were obtained from Aldrich Chemical (Milwaukee, WI). All materials were used without further purification. All organic extracts were dried using anhydrous magnesium sulfate (Aldrich Chemical).
2.2 Characterization
1H and 13C NMR spectra were recorded using a JEOL JNM-ECP 400 spectrometer operating at 400.13 and 100.77 MHz, respectively. The spectrometer used a 5-mm broadband inverse Z-gradient probe in DMSO-d6 solvent (Cambridge Isotope Laboratories, Andover, MA) as solvent. Each spectrum was Fourier-transformed, phase-corrected, and integrated using a MestRe-C 2.3a software (Magnetic Resonance Companion, Santiago de Compostela, Spain). FTIR spectra were recorded neat on a Thermo Nicolet Nexus 470 FTIR system (Madison, WI) with a Smart ARK accessory containing a 45 ZeSe trough in a scanning range of 650–4000 cm−1 for 32 scans at a spectral resolution of 4 cm−1.
2.3 Low temperature operability
The pour point (PP) is defined as the lowest temperature at which the sample still pours from a tilted jar. This method is routinely used to determine the low temperature flow properties of fluids. PP values were measured according to the ASTM D5949 method (ASTM Standard D5949) using a Phase Technology Analyzer, Model PSA-70 S (Hammersmith Gate, Richmond, BC, Canada). Each sample was run in triplicate and the average values are reported rounded to the nearest whole degree. For a greater degree of accuracy, PP measurements were done with a resolution of 1 °C instead of the specified 3 °C increment. Generally, materials with lower PP exhibit improved fluidity at low temperatures than those with higher PP.
2.4 Flash point values
The flash point (FP) is defined as the minimum temperature at which the liquid produces a sufficient concentration of vapor above it that it forms an ignitable mixture with air. The lower the flash point is, the greater the fire hazard. Flash point determination was run according to the American National Standard Method using a Tag Closed Tester (ASTM D 56-79) (ASTM Standard D 56-79). Each sample was run in triplicate and the reported average values are rounded to the nearest whole degree.
2.5 Viscosity index measurements
Automated HV M472 multi range viscometer tubes obtained from Walter Herzog (Germany) were used to measure viscosity. Measurements were run in a Temp-Trol (Precision Scientific, Chicago, IL, USA) viscometer bath set at both 40.0 and 100.0 °C. The viscosity and viscosity index were calculated using ASTM methods D 445-97 (ASTM D 445-97) and ASTM D 2270-93 (ASTM D 2270-93), respectively. The measurements were taken in triplicate and the average values were reported.
2.6 Oxidation stability
Pressurized differential scanning calorimetry (PDSC) experiments were achieved using a DSC 2910 thermal analyzer from TA Instruments (Newcastle, DE). Typically, a 2-μL sample, resulting in a film thickness of less than 1 mm, was placed in an aluminum pan hermetically sealed with a pinhole lid. This was oxidized in the presence of dry air (Gateway Airgas, St Louis, MO), which was pressurized in the module at a constant pressure of 1378.95 kPa (200 psi). Each experiment utilized a 10 °C min−1 heating rate to get from 50 to 350 °C. The oxidation onset (OT, °C) and signal maximum temperatures (SMT, °C) were calculated from a plot of heat flow (W/g) versus temperature for each experiment. Each sample was run in triplicate and the reported average values are rounded to the nearest whole degree (Table 1).
2.7 Synthesis
2.7.1 Epoxidized oleic acid (EOA, 1)
Hydrogen peroxide solution (30% in H2O, 8.0 mL) was added slowly into a stirred solution of oleic acid (OA) (90%, 15 g) in formic acid (88%, 14 mL) at 4 °C (ice bath). The reaction proceeded at room temperature with vigorous stirring (900 rpm) until the formation of a powdery solid was noticed in the reaction vessel 2–5 h. The solid was collected via vacuum filtration, washed with H2O (chilled, 3 × 10 mL), and placed under high vacuum for 12 h, yielding epoxidized oleic acid (EOA) as a colorless, powdery solid.
2.7.2 9-Hydroxy-10-acyloxyoctadecanoic acid (HYAODA, 2–8)
To a mixture of EOA (31 g) was added 5 g of p-toluenesulfonic acid (PTSA), toluene (70 mL) and fatty acids (6 g) over 1.5 h in order to keep the reaction mixture temperature under 70–80 °C. The reaction mixture was subsequently heated to 90–100 °C and refluxed for 3 h. After reaction termination, the heating was stopped and the mixture was left to stand overnight at ambient room temperature. The mixture was washed with water the next day. The organic layer was dried over anhydrous magnesium sulfate and the solvent was removed using a vacuum evaporator.
2.7.3 Butyl 9-hydroxy-10-acyloxyoctadecanoate (BHYAOD, 9–15)
Sulfuric acid (conc. H2SO4, 10 mol%) was added into a stirred suspension of HYAODA (3.35 mmol) in butanol (3.35 mL). The suspension was heated with stirring at 60 °C for 10 h. Hexanes (5 mL) was then added. The solution was washed with NaHCO3 (sat. aq., 1 × 0.5 mL) and brine (2 × 1 mL), dried using MgSO4, filtered, concentrated in vacuo and placed for 6 h under vacuum to yield the desired product.
2.7.4 Butyl 9-(decanoyloxy)-10-(acyloxy)octadecanoate (BDOAOD, 16–22)
The synthetic scheme for the triesters is shown in Fig. 1. Appropriate amounts of BHYAOD, pyridine and CCl4 were weighed out and put into a 500-mL three-necked flask equipped with a cooler, dropping funnel and thermometer. The mixture was heated to 50 °C with suitable aliquots of decanoyl chloride added over 1 h. The reaction mixture was subsequently refluxed for 4 h. Upon completion, the mixture stood overnight at an ambient temperature. After washing with water, the solvent extract was dried over anhydrous sodium sulfate, further filtered and vacuum distilled to remove the residual solvent.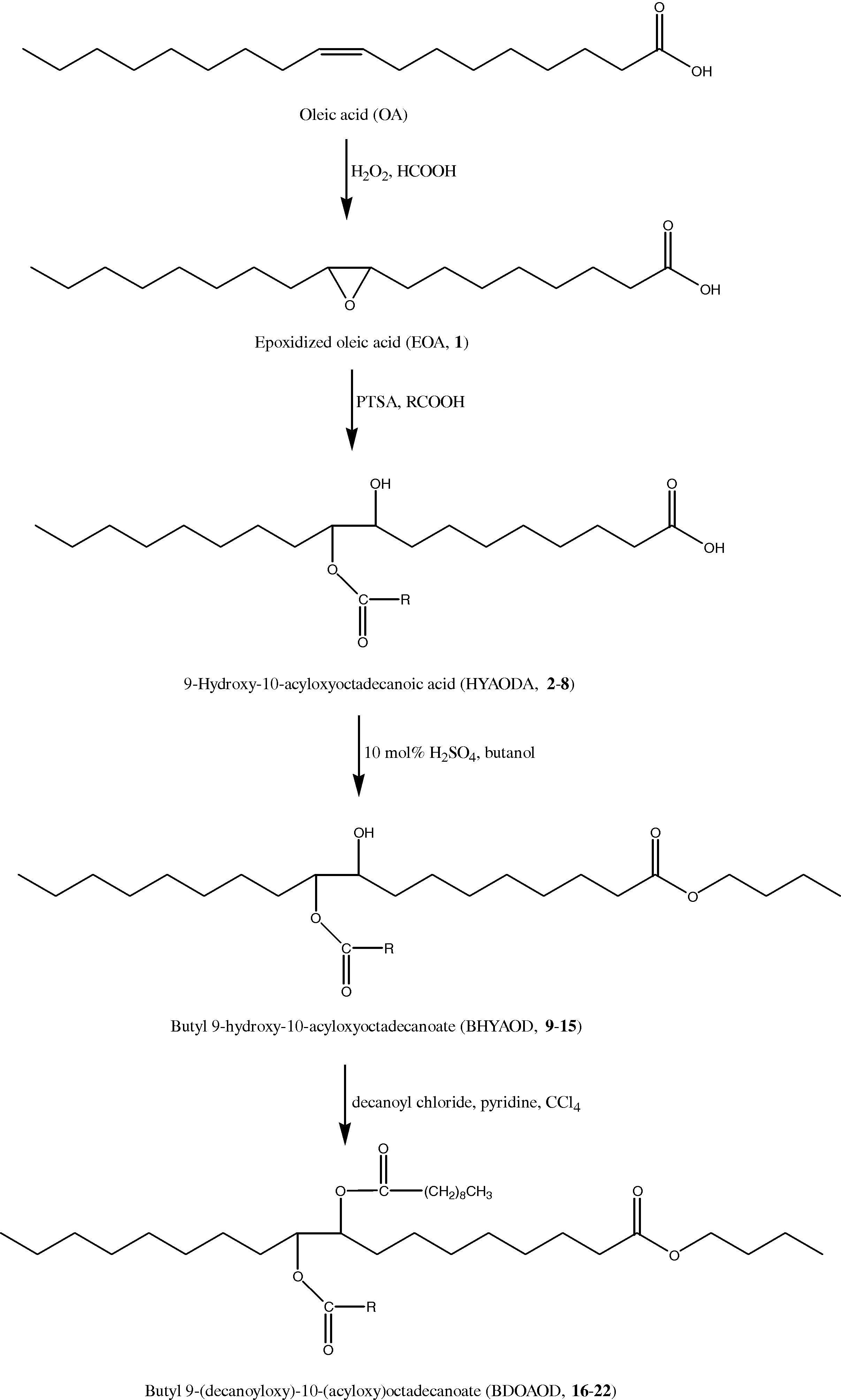
Triesters formation. RCOOH = octanoic, nonanoic, lauric, myristic, palmitic, stearic and behenic acids.
3 Results and discussion
The preparation of butyl 9-(decanoyloxy)-10-(acyloxy)octadecanoate 16–22 from EOA is an effective way of introducing branching onto the fatty acid (FA) chains of plant oils. This reaction is carried out in four stages and the final products have significantly improved oxidative stability and low-temperature property compared with the starting materials (Fig. 1). The straightforward epoxidation of oleic acid was closely monitored to avoid the synthesis of the undesired 9,10-dihydroxyoctadecanoate, which will form if the reaction temperature is elevated or the reaction is allowed to progress for too long.
The removal of unsaturation in the oleic acid by converting them to epoxy-groups 1 improves the oxidative stability. It has already been established that the presence of multiple double bonds in the plant oil FA chains accelerates oxidative degradation (Hwang et al., 2003). However, the low-temperature fluidity of 1 is poor and found to solidify at 0 °C (see Table 1). This would limit the application of plant oil at low operating temperature especially as automotive and industrial fluids. A suitable approach to improve the low-temperature flow behavior of 1 is to attach branching sites at the epoxy carbons. This was achieved by careful ring opening to obtain the 9-hydroxy-10-acyloxyoctadecanoic acid (HYAODA) products 2–8. Then, esterification of these products was carried out using butanol and sulfuric acid as catalyst to yield butyl 9-hydroxy-10-acyloxyoctadecanoate (BHYAOD) 9–15. The seven prepared butyl esters were used as feed for the synthesis of modified triester-derivatives by acetylation in an aprotic solvent. The yields are summarized in Table 1.
In the FTIR spectra of compounds 2–22, the absorption due to the epoxy group (822 and 842 cm−1) is not observed. This fact suggests that 1 undergoes complete ring opening under the reaction condition. Bands representing C⚌O groups (725, 1743 cm−1), CH3 groups (1373–1461 cm−1), OH groups (3475–3440 cm−1) and also C–O–C bands in esters (998–1100 cm−1) are clearly visible in the spectra (Salimon and Salih, 2010).
All synthesized compounds were verified by 1H and 13C NMR spectroscopy. Significant signals in the 1H spectrum of EOA 1 between 2.5 and 2.7 ppm correspond to quaternary carbons of the oxiran ring and the doublet in the 13C spectrum between 56.89 and 56.90 ppm corresponds to carbons of the oxirane ring. Furthermore, 1H spectrum of EOA showed a singlet signal at 9.20 ppm due to the OH group. All signals in the area around 9.15–9.27 ppm, representing OH groups, and the bands at 2.05–3.66 ppm, corresponding to –CH2– groups, are present in the 1H spectra of monoesters, HYAODA 2–8. The 1H spectra of synthesized diesters, BHYAOD 9–15, and triesters, BDOAOD 16–22, consist of signals of low intensity at about 9.22–9.40 ppm and 2.10–3.65 ppm. Broad lines at 1.40–1.78 ppm represent the CH2 groups’ hydrogen peaks. In the 13C NMR spectra significant bands at about 174 ppm are present, which exhibit the characteristic signals attributed to the ester groups (Sliverstien et al., 2005).
Physicochemical properties of prepared compounds are summarized in Table 1. The ability of a substance to remain as a liquid at low temperatures is an important attribute for a number of industrial materials, such as biolubricants, surfactants and fuels. The cold flow property of plant oils is extremely poor and this limits their use at low operating temperature especially as automotive and industrial fluids. Plant oils have a tendency to form macrocrystalline structures at low temperature through uniform stacking of the ‘bend’ triglyceride backbone. Such macrocrystals restrict the easy flow of the system due to loss of kinetic energy of individual molecules during self-stacking. Cold flow properties of these samples were determined using their pour points. In practice, the usable liquid range is limited by the pour point (PP) at low temperatures and the flash point at high temperatures. The PP should be low to ensure that the lubricant is pump-able when the equipment is started from extremely low temperatures (Salimon et al., 2010). The flash point should be high to allow the safe operation and minimum volatilization at the maximum operating temperature, Table 1. For the most demanding applications, such as aviation jet engine lubricants, an effective liquid range over 300 °C may be required. The EOA, synthetic mono-, di- and triesters described above were screened for low-temperature behavior through determination of their pour point (PP).
An improvement in the cold flow behavior of diesters BHYAOD and triesters BDOAOD was obtained over that of their monoester precursors HYAODA. Actually there are two reasons for this behavior. The first reason is that the presence of a side chain attached to the FA backbone does not allow individual molecules to come close for easy stacking due to steric interactions. This results in the formation of microcrystalline structures rather than macro structures. At lower temperatures, such microcrystalline structures can easily tumble and glide over one another resulting in better fluidity of the total matrix. Secondly, the lack of one hydroxyl group in diesters and then the absence of it in triester structures mean that the number of hydrogen bonds decrease, which could cause the molecules to stack together.
The efficiency of the biolubricant in reducing friction and wear is greatly influenced by its viscosity. Generally, viscosity-temperature charts are available, making a good choice of a biolubricant operation temperature. The viscosity of a biolubricant is its tendency to resist flow. A biolubricant oil of high viscosity flows very slowly. The viscosity must always be high enough to keep a good oil film between the moving parts. Otherwise, friction will increase, resulting in power loss and rapid wear on the parts. The viscosity index, commonly designated as VI, is an arbitrary numbering scale that indicates the changes in oil viscosity with changes in temperature. A low index means a steep slope of the curve, or a great variation of viscosity with change in temperature; high index means a flatter slope, or lesser variation of viscosity with the same changes in temperature. Increased viscosity index (VI) of triesters is the result of their higher molar weight, and especially the altered structure of their molecules. The VI values are high, characteristic for oils of ester type (Table 1). HYOODA: 9-hydroxy-10-octyloxyoctadecanoic acid, HYNODA: 9-hydroxy-10-nonanoxyoctadecanoic acid, HYLODA: 9-hydroxy-10-lauroxyoctadecanoic acid, HYMODA: 9-hydroxy-10-myristoxyoctadecanoic acid, HYPODA: 9-hydroxy-10-palmitoxyoctadecanoic acid, HYSODA: 9-hydroxy-10-stearoxyoctadecanoic acid, HYBODA: 9-hydroxy-10-behenoxyoctadecanoic acid, BHYOOD: butyl 9-hydroxy-10-octyloctadecanoate, BHYNOD: butyl 9-hydroxy-10-nonanoxyoctadecanoate, BHYLOD: butyl 9-hydroxy-10-lauroxyoctadecanoate, BHYMOD: butyl 9-hydroxy-10-myristoxyoctadecanoate, BHYPOD: butyl 9-hydroxy-10-palmitoxyoctadecanoate, BHYSOD: butyl 9-hydroxy-10-stearoxyoctadecanoate, BHYBOD: butyl 9-hydroxy-10-behenoxyoctadecanoate, BDOOOD: butyl 9-(decanoyloxy)-10-(octyloxy)octadecanoate, BDONOD: butyl 9-(decanoyloxy)-10-(nonanoxy)octadecanoate, BDOLOD: butyl 9-(decanoyloxy)-10-(lauroxy)octadecanoate, BDOMOD: butyl 9-(decanoyloxy)-10-(myristoxy)octadecanoate, BDOPOD: butyl 9-(decanoyloxy)-10-(palmitoxy)octadecanoate, BDOSOD: butyl 9-(decanoyloxy)-10-(stearoxy)octadecanoate, BDOBOD: butyl 9-(decanoyloxy)-10-(behenoxy)octadecanoate.
Samples
Pour pointa (°C)
Flash pointa (°C)
Viscosity indexa
OTa (°C)
SMTa (°C)
Percentage yield
EOA
0
113
45
75
164
91
Monoesters 2–8
HYOODA
−20
250
71
113
123
70
HYNODA
−30
305
80
101
256
63
HYLODA
−33
176
84
91
189
80
HYMODA
−35
199
89
83
213
56
HYPODA
−39
123
93
76
209
92
HYSODA
−41
194
102
70
243
85
HYBODA
−43
232
110
64
175
76
Diesters 9–15
BHYOOD
−24
115
82
152
95
65
BHYNOD
−33
145
89
146
112
73
BHYLOD
−36
162
91
134
145
80
BHYMOD
−39
209
96
101
160
83
BHYPOD
−42
120
101
93
135
52
BHYSOD
−44
165
110
89
189
76
BHYBOD
−46
187
122
80
201
81
Triesters 16–22
BDOOOD
−26
123
134
173
118
55
BDONOD
−35
145
151
169
142
69
BDOLOD
−38
110
162
162
131
71
BDOMOD
−41
157
170
150
152
82
BDOPOD
−43
173
183
125
167
86
BDOSOD
−45
180
190
103
189
73
BDOBOD
−47
189
202
91
162
65
The ability of a substance to resist oxidative degradation is another important aspect of biolubricants. Therefore, EOA, HYAODA, BHYAOD and BDOAOD were screened for oxidation stability using PDSC through determination of OT and SMT. PDSC is an effective method for measuring oxidation stability of oleochemicals in an accelerated mode (Du et al., 2002). The OT is the temperature at which a rapid increase in the rate of oxidation is observed at a constant, high pressure (200 psi). A high OT would suggest high oxidation stability of the material. The SMT is the temperature at which maximum heat output is noted from the sample during oxidative degradation. A higher SMT does not necessarily correlate with improved oxidation stability. Both OT and SMT were calculated from a plot of heat flow (W/g) versus temperature that was generated by the sample upon degradation and, by definition, SMT is greater than OT.
In the present study, as the chain length of the mid chain ester is decreased, a corresponding improvement in oxidation stability was observed, which is because longer chains are more susceptible to oxidative cleavage than shorter chains. These results are in agreement with other studies on synthetic esters (Randals, 1999). For example, when comparing HYAODA monoesters, BHYAOD diesters and BDOAOD triesters, an improvement in OT was noticed as the mid chain ester length (R) was decreased (Table 1).
4 Conclusion
The results show that under some conditions, triester products have better biolubricant properties which enable them to outperform their precursors. Additionally, they maintain the advantages inherent from their biobased nature. These products remain a viable option for application in the biolubricant industry. The following conclusions can be drawn:
Increasing the chain length of the mid chain ester had a positive influence on the low temperature properties of the synthesized esters because they create a steric barrier around the individual molecules and inhibits crystallization, resulting in lower PP.
The trends for PP were opposite to those of OT. For example, increasing chain length was beneficial to PP, but detrimental to OT.
It is evident that hydrogen bonding was a critical parameter influencing the low temperature properties and oxidation stability of the synthesized esters. An increase in hydrogen bond amount will lead to an increase in PP and a decrease in the oxidation stability of these compounds.
Removal of the unstable double bonds from fatty acid acyls, increased molar weight and change in the molecular structure result in an increased VI of the synthesized esters.
Acknowledgments
We are grateful to Universiti Kebangsaan Malaysia for funding (“codes UKM-GUP-NBT-08-27-113”, “UKM-GGPM-NBT-164-2010”, UKM-ST-06-FRGS0113-2009 and “UKM-OUP-NBT-29-150/2010”). We would like to thank the support staff from the School of Chemical Sciences and Food Technology and the faculty of Science and Technology from Universiti Kebangsaan Malaysia. Also, we offer special thanks to SRF and IIE.
References
- ASTM Standard D5949: Standard test method for pour point of petroleum (automatic pressure pulsing method). ASTM, West Conshohocken, PA, USA.
- ASTM Standard D 56-79: Standard test method for flash point of liquids with a viscosity less than, 45 Saybolt Universal Seconds (SUS) at 37.8 °C (that don’t contain suspended solids and don’t tend to form a surface film under test).
- ASTM D 445-97: Standard test method for kinematic viscosity of transparent and opaque liquids. ASTM, West Conshohocken, PA, USA.
- ASTM D 2270-93: Standard practice for calculating viscosity index from kinematic viscosity at 40 and 100 °C. ASTM, West Conshohocken, PA, USA.
- Ecotribology: environmentally acceptable tribological practices. Tribol. Int.. 2006;39:728-733.
- [Google Scholar]
- Antioxidation synergism between ZnDTC and ZnDDP in mineral oil. Tribol. Lett.. 2002;13:21-27.
- [Google Scholar]
- Vegetable oil-based lubricants – a review of oxidation. Tribol. Int.. 2007;40:1035-1046.
- [Google Scholar]
- Guzman, D., 2002. Vegetable oil in lubricants and additives slated for strong growth. Chemical Market Reporter, June 10, 1–2.
- Preparation and properties of lubricant basestocks from epoxidized soybean oil and 2-ethylhexanol. J. Am. Oil Chem. Soc.. 2003;80:811-815.
- [Google Scholar]
- Kodali, D.R., Nivens, S., 2001. 2nd World Tribology Congress, Abstract of Papers, Vienna, p. 235.
- Mixtures of vegetable oils and di-2-ethylhexyl-sebacate as lubricants. J. Syn. Lubr.. 2006;23:185-196.
- [Google Scholar]
- Rudnick L.R., Shubkin R.L., eds. Esters in synthetic lubricants and high-performance functional fluids. New York: Marcel Dekker; 1999. p. :63-102.
- Modification of epoxidized ricinoleic acid for biolubricant base oil with improved flash and pour points. Asian J. Chem.. 2010;22:5468-5476.
- [Google Scholar]
- Biolubricants: raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol.. 2010;112:519-530.
- [Google Scholar]
- Transesterification of vegetable oil: a review. J. Braz. Chem. Soc.. 1998;9:199-210.
- [Google Scholar]
- Spectrometric identification of organic compounds (7th ed.). New York: John-Wiley; 2005. p. 25–27







