Translate this page into:
Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus Say larvae (Diptera: Culicidae)
*Corresponding author ralmehmadi@kau.edu.sa (R.M. Al-Mehmadi), d_almehmadi@yahoo.com (R.M. Al-Mehmadi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Extracts from Melia azedarach L. (Meliaceae) were effective against third instar larvae of Culex quinquefasciatus (Say) in Saudi Arabia, using crude extract obtained in ethanolic solution from King Saud University. Toxicity was varied according to the concentration and period of exposure. We investigated the effect of the LC50 on midgut and gastric caecae of 3rd larval instar of Cx. quinquefasciatus, the plant extract causing serious damage to the epithelial columnar cells. Light and electron microscopic observations revealed, by time 6, 12, 24 and 48 h, increasing damages of the larvae midgut epithelium. The most characteristic effects were midgut columnar cell vacuolization, microvilli damages, epithelium cell contents passing into the midgut lumen and finally the cell death. This article is the first report of the histopathological effects of the M. azedarach as a bioinsecticide in the midgut of Cx. quinquefasciatus larvae and the data obtained may contribute to a better understanding of the mode of action of this plant extract used as a bioinsecticide against Cx. quinquefasciatus larvae.
Keywords
Melia azedarach
Culex quinquefasciatus
Midgut
Histology
1 Introduction
The biological control of immature stages now appears to be the most powerful means of reducing target populations of Culicidae and other dipteran pests. As a result of continuous application of chemical pesticides, resistance was a squired by insect pests besides residue contamination of human foods, mammalian toxicity and pollution of the environment. Numerous secondary compounds from plants are being studied for use as new effective, eco-friendly biopesticides (Pathak and Dixit, 1988; Chockalingam et al., 1990; Govindachari et al., 1996; Jayaprakasha et al., 1997). Muthukrishnan and Puspalatha (2001) evaluated the larvicidal activity of extracts from Calophyllum inophyllum (Clusiaceae), Rhinacanthus nasutus (Acanthaceae), Solanum suratense (Solanaceae) and Samadera indica (Simaroubaceae), Myriophyllum spicatum (Haloragaceae) against Anopheles stephensi. Several indigenous plants viz., Ocimum basilicum, Ocimum santum, Azadirachta indica, Lantana camera, Vitex negundo and Cleome viscosa were studied for their larvicidal action on the field which collected fourth instar larva of Cx. quinquefasciatus (Kalyanasundaram and Dos, 1985). Studies have shown the potential of plants for use in Cx. quinquefasciatus control, such as Piper nigrum (Chahad and Boof 1994), Azadirachta indica, Rhazya sticta and Syzygium aromaticum (Mishra et al., 1995; Su and Mulla, 1998; Das et al., 1999; El-Hag et al., 1999), Agave americana and Kaempferia galanga (Dharmshaktu et al., 1987; Choochote et al., 1999, Atriplex halimus (El-Gougary, 1998; Massoud and Labib, 2000) and Commiphora molmol (Pitasawat et al., 1998). Persian lilac tree and also known as ‘cinnamon’ is related to Meliaceae trees. Effects of M. azedarach extracts on many insects have been already reported (Saxena et al., 1984; Schmidt et al., 1998; Juan et al., 2000; Carpinella et al., 2003). The Meliaceae plant family has been known as a potential source for insecticide properties. Extracts from the neem and other plants seeds and leaves have shown excellent insecticidal properties against fecundity and fertility of mosquito vector and were at the same time very eco-friendly (Schmutterer, 1990; Senthil Nathan et al., 2005). The efficacy of these neem products on mosquitoes was established (Zebitz, 1984; Schmutterer, 1990; Murugan et al., 1996). The histopathological changes in treated insects with alternative Insect control as a toxic action were previously investigated (Charles, 1987; Davidson and Titus,1987; Singh and Gil, 1988; Silva-Filha and Peixoto, 2003), and with botanical insecticides were also studied (Nasiruddin and Mordue, 1993).
The present study was conducted to evaluate the mosquito larvicidal activity of Melia azedarach ethanol extracts against Cx. quinquefasciatus larvae. Larval susceptibility to M. azedarach was also compared at the histopathological level to elucidate the effects of this potential bioinsecticide.
2 Materials and methods
2.1 Rearing technique
Laboratory sensitive strain of Cx. quinquefasciatus mosquitoes was obtained from susceptible reared strain in College of Food and Agricultural Science, King Saud University, the larvae used in the tests were reared in a plastic cup with water from the public water supply, under the standard techniques at 27 ± 2 °C and 70 ± 5% R.H. and 12 h photoperiod. Larvae were reared in dechlorinated water and fed daily on tetramine (tropical fish food). Adults were maintained on a 10% sugar solution and females were also fed on chicken. Third instar was used in the bioassay tests.
2.2 Preparation of stock solution
Ethanolic extract of whole plant of M. azedarach was obtained from Pharmacy College, King Saud University, as a dark green color crude extract. This crude extract was used to prepare stock solution. The known amount (10 mg/l) of filtered crude extract obtained from the above-mentioned source was serially diluted to obtain the desired concentration. The stock solution was serially diluted to prepare the test solutions of 0.50, 1.00, 1.25, 1.50, 1.75 and 2.00 mg/l. One drop of emulsifier (Tween 20, Sigma Chemical Company) was added with the extract to ensure complete solubility of the solutions in water.
2.3 Bioassays and larval mortality
Ten larvae were pipette into each 20 ml volume (in four replicates) and observed for a maximum of 48 h, when mortality was recorded. Larvae were considered dead or moribund if they stopped moving for a prolonged period even after gentle probing with a small spatula, as described in the World Health Organization's technical report series. A minimum of 40 larvae/concentration was used for all the experiments (WHO/CDS/WHOPES/GCDPP/2005.13). Larvae maintained in distilled water were used as a control. The LC50 and LC90 were calculated using probit analysis (Finney, 1971). Percentage mortality in the treatments was corrected when necessary for mortality in the controls using Abbot‘s (1925) formula.
2.4 Histological studies
For the Histological tests, treated and untreated newly ecdysed third instar larvae of Cx. quinquefasciatus were isolated from the standard laboratory colony reared on fed which were incorporated with the LC50 of M. azedarach after 24 h = (1.035 mg/l). Only live larvae were examined. Then they were fixed in bouins solution (after 6, 12, 24 and 48 h from exposures) for 24 h. After dehydration in a graded ethanol series, the material was embedded and cut with glass knives in a rotary microtome. The sections were stained with haematoxylin-eosin, analyzed, and photographed with a photomicroscope. After 24 and 48 h for electron microscopic studies, the midgut was fixed in a solution containing 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3), post fixed in 1% osmium tetroxide solution in the same buffer, dehydrated in a graded acetone solution, and embedded. Ultra-thin sections were stained with uranyl acetate and lead citrate before examination.
3 Results
Effect of M. azedarach extract on the 3rd instar larvae increased with an increase of concentration. The LC50 after 24 h was 1.035 mg/l. The LC50 after 48 h was 0.75 mg/l. Regression analysis showed a concentration-dependent significant correlation of the plant extract with larval mortality. Strong correlation between concentration and mortality (r) was 0.963 and 0.97 after 24 and 48 h, respectively (Table 1) (see Fig. 1). Index compare with M. azedarach after 48 h. Resistance Ratio (RR) compared with M. azedarach after 48 h.
No. larvae treated
Exposure time (h)
LC50 (mg/l)
Lower limit
Upper limit
Index
RR
Slope ± SE
Chi-square
LC90
40
M. azedarach after 48 h
0.754
0.646
0.861
100.0
1.00
3.236 ± 0.372
5.15
1.876
40
M. azedarach after 24 h
1.035
0.935
1.124
72.85
1.373
5.477 ± 00.60
6.01
1.773
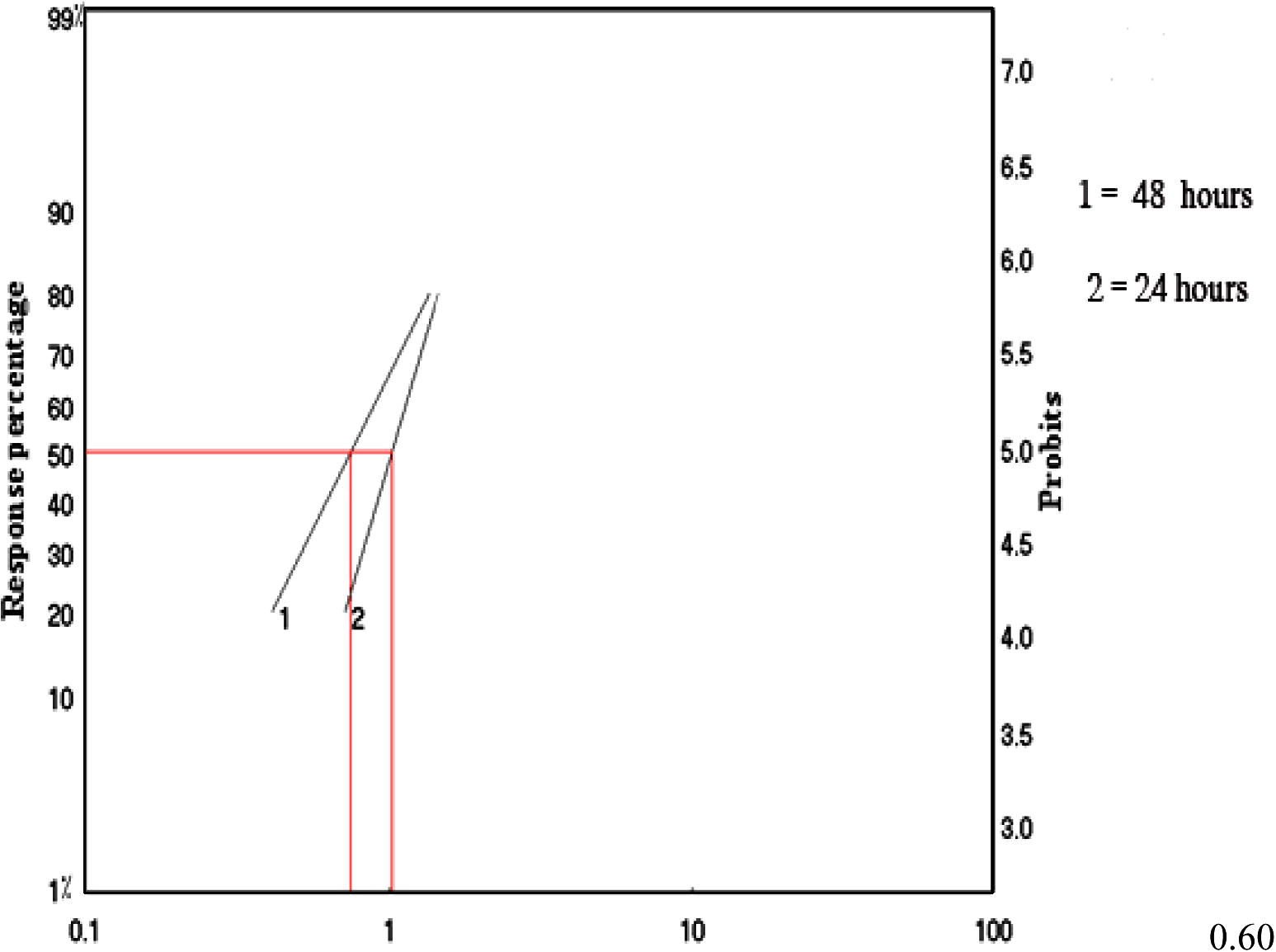
Efficacy of M. azedarach.extracts on 3rd instar larvae of Cx. quinquefasciatus mosquitoes at different periods of exposure.
The first aspect investigated in this work was the histological structure of midgut epithelial cells from untreated larvae. The midgut of dipteran larvae has been subdivided into two different regions, each including one characteristic cell type (Rey et al., 1999). The anterior midgut included flatter cells with clear cytoplasm (clear cells: Clements, 1992), extending along one-third of the midgut. Depending on their stage of development, clear cells displayed different degrees of apical swelling into the gut lumen, reducing intercellular contacts with the neighboring cells and degeneration of the nuclei and brush border, as shown, for example, in control Cx. quinquefasciatus (Fig. 2A). The posterior midgut was characterized by tall epithelial cells (dark cells: Clements, 1992). Dark cells show normal intercellular contacts along the whole lateral plasma membranes, normal nuclei, a well-developed brush border, and a normal adhesive basement membrane, as observed in control sections (Fig. 2B). When treated with M. azedarach extract, all larvae developed dramatic lesions, affecting mainly the midgut epithelium and secondarily the caeca. Histopathological effects differed qualitatively according to their localization along the midgut and quantitatively according to the duration of the treatment.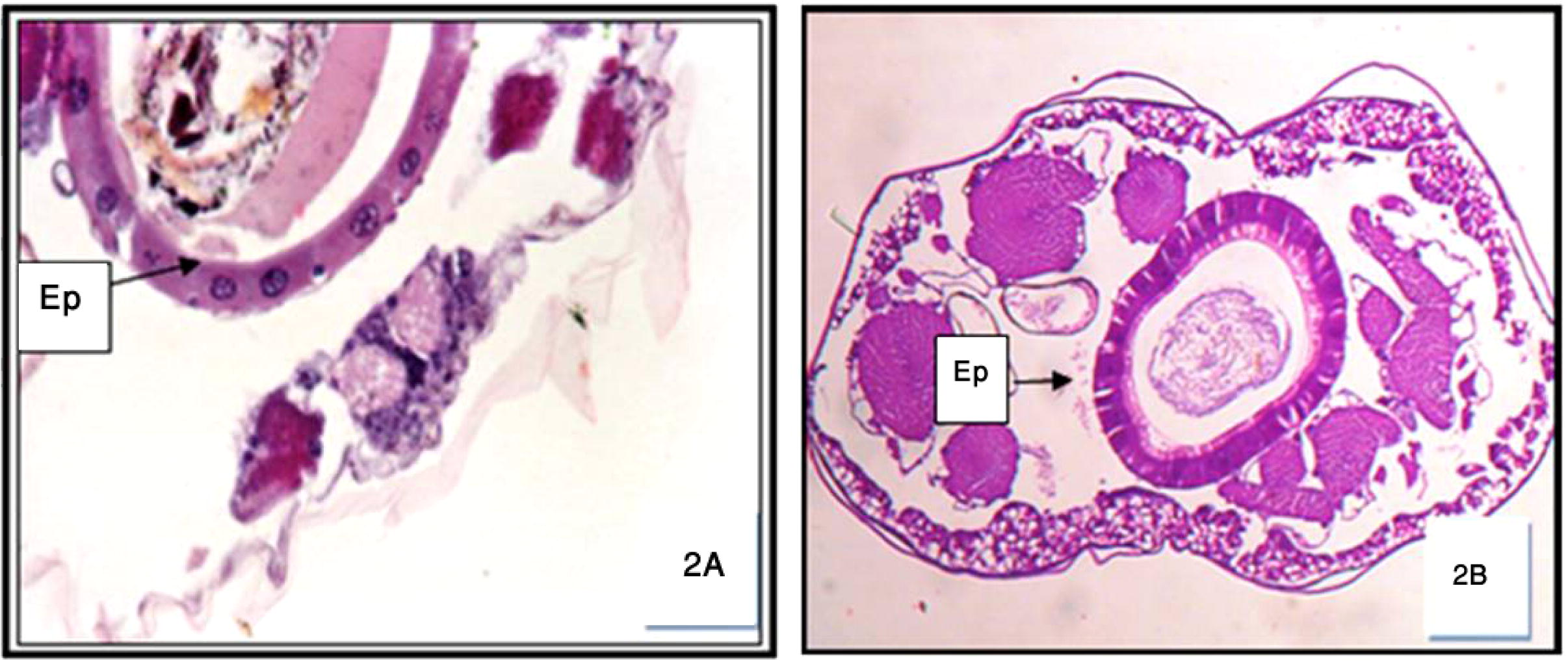
Cross-section in the anterior (A) and posterior (B) regions of normal untreated Cx. quinquefasciatus 3rd instar larva showing the foregut-epithelia cells (Ep.). 200×.
3.1 After 6 h of treatment
Cells of the anterior midgut show hardly any morphological deviation from normal ones, through slightly apical degenerated (lysis) of clear cells and little disruption of the lateral junctional complexes. Compared to controls, acceleration of the lysis of clear cells was perceptible at the level of their brush border, basal membrane, nucleus, and cytoplasmic organelles, before their bursting into the gut lumen. At the same time, partial lysis of the posterior midgut began through local detachment among small groups of dark cells bearing a dilated basal membrane, destruction of the peritrophic membrane; the cells were elongated (Figs. 3 and 4A and B).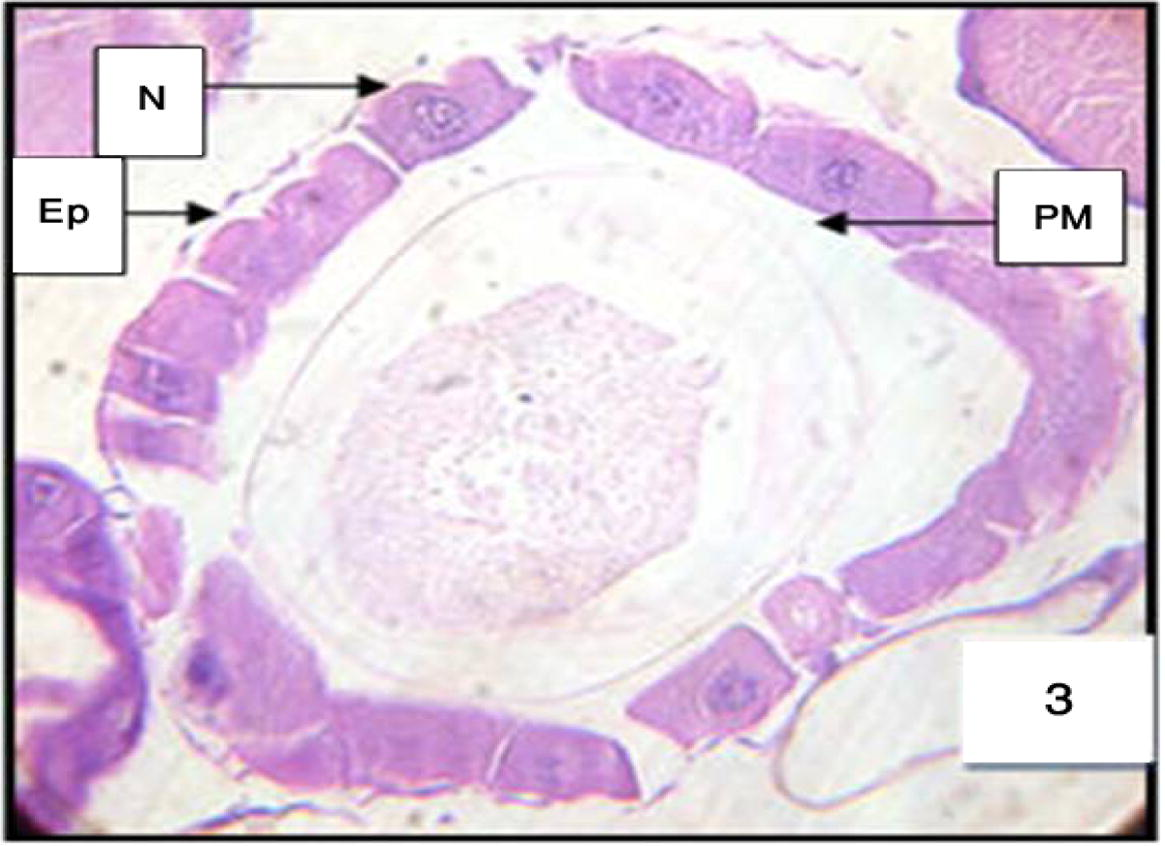
Anterior midgut of 3rd instar larvae of Cx. quinquefasciatus treated with LC50 of M. azedarach extract, showing the effect after 6 h of exposure. Epithelial cells (EP), peritrophic membrane (PM) and nucleus (N). 400×.
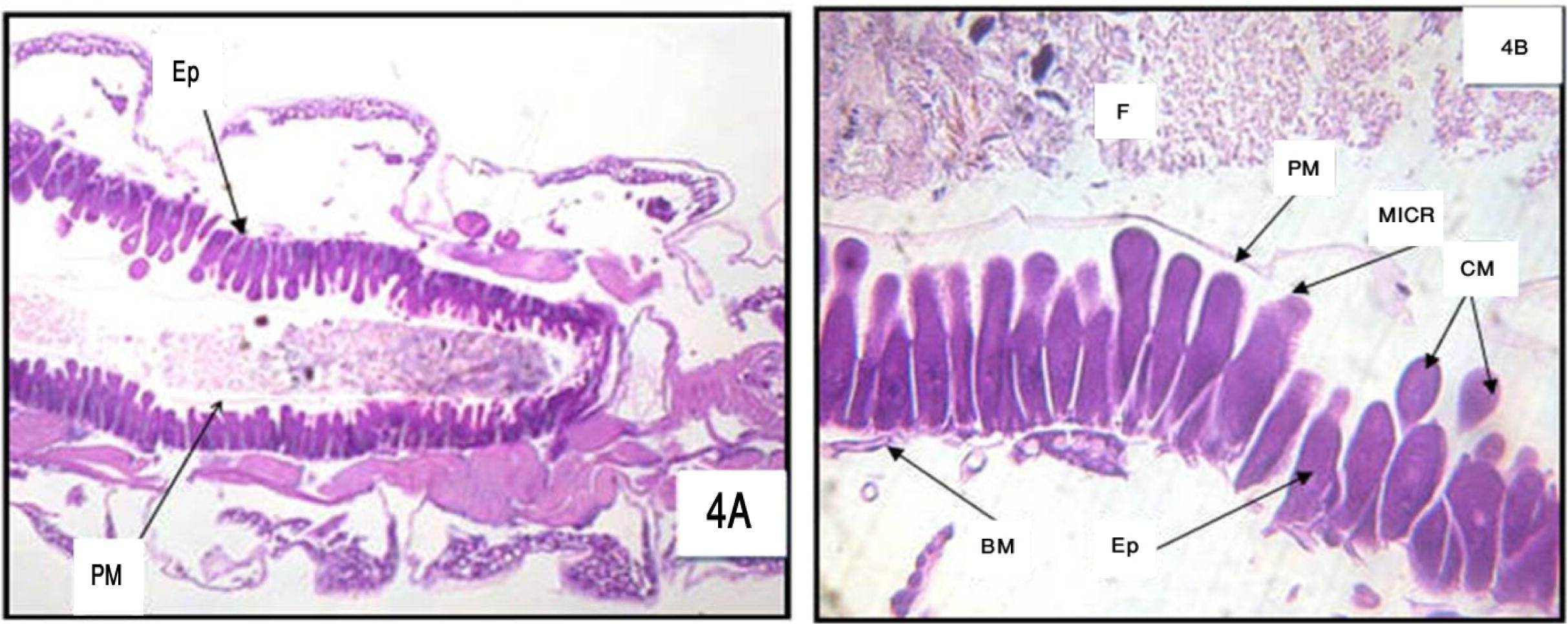
(A) and (B) Longitudinal section in the posterior region of midgut of 3rd larval instar of Cx. quinquefasciatus treated with LC50 of M. azedarach after 6 h (A = 100× and B = 400×) Epithelial cells (Ep), peritrophic membrane (PM), food bolus (FB), basement membrane (BM), microvilli (MICR) and cytoplasm masses (CM).
3.2 After 12 h of treatment
The lysis of the anterior midgut progressed through swollen clear cells, vacuoles, nuclear degenerated (Fig. 5). In the posterior midgut, disruption of the junctional complexes among dark cells progressed apically, together with their cytoplasmic and nuclear lysis, their local detachment from the basal lamina, and the degeneration of the microvilli (Fig. 6).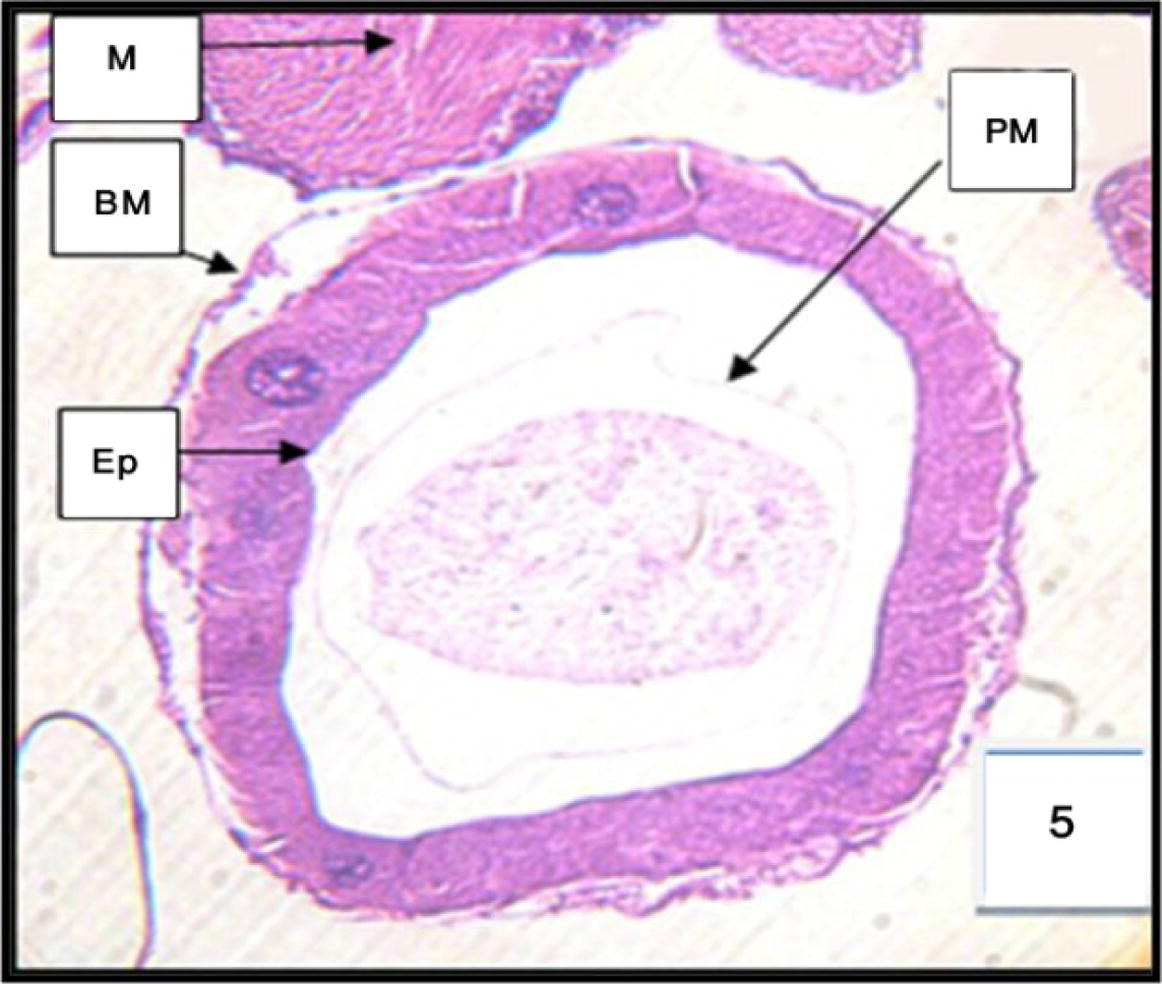
Cross section through the anterior midgut of 3rd instar larvae of Cx. quinquefasciatus treated with LC50 of M. azedarach extract, showing the effect after 12 h of exposure. 400×. 1-Epithelial cells of anterior region (EP.), muscles (M), basement membrane (BM), and peritrophic membrane (PM).
Cross section in the posterior region of midgut in 3rd Cx. quinquefasciatus larval instar treated with LC50 of M. azedarach extract, showing the effect after 12 h of exposure. Epithelial cells (EP) and peritrophic membrane (PM). 400×.
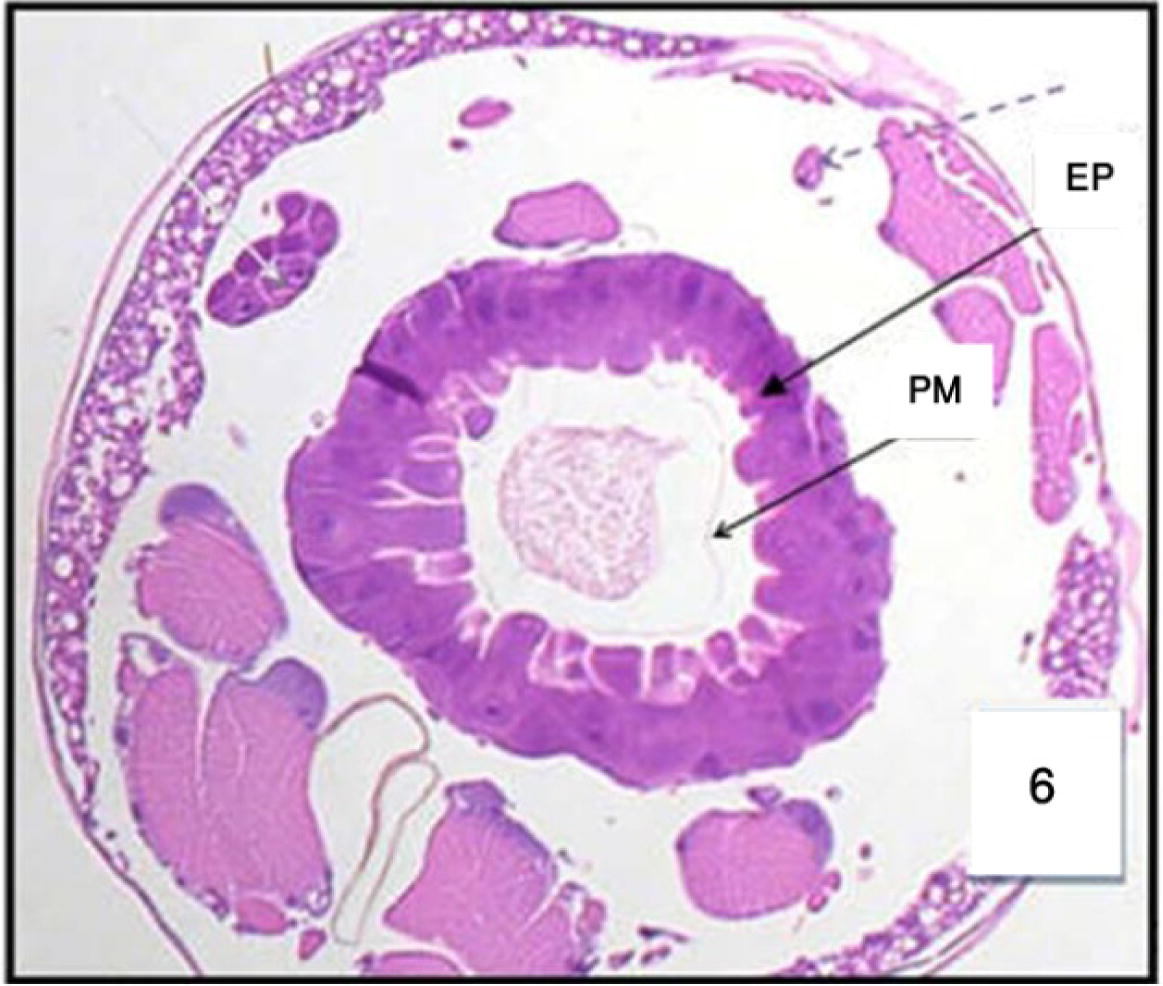
3.3 After 24 h of treatment
The anterior midgut was almost globular structure bulging from their free surface. The other kinds of cell appeared short and polygonal; few cells of the epithelium exhibited a brush border, some with normal nuclei, a well-developed brush border. As shown in ultrastructure the midgut of non-treated larvae possesses a well-preserved layer of epithelial cells, the surrounding basal lamina was underneath the basal labyrinth. The gut lumen was lined by a brush border membrane composed of numerous and regularly placed microvilli (Figs. 16 and 17), as observed in control sections of the posterior midgut progressed both extracellular, through detachment of dark cells from each other, and from the basal lamina (Figs. 7 and 8 and ultrastructural sections Figs. 18 and 19), and intracellular, through cell bursting.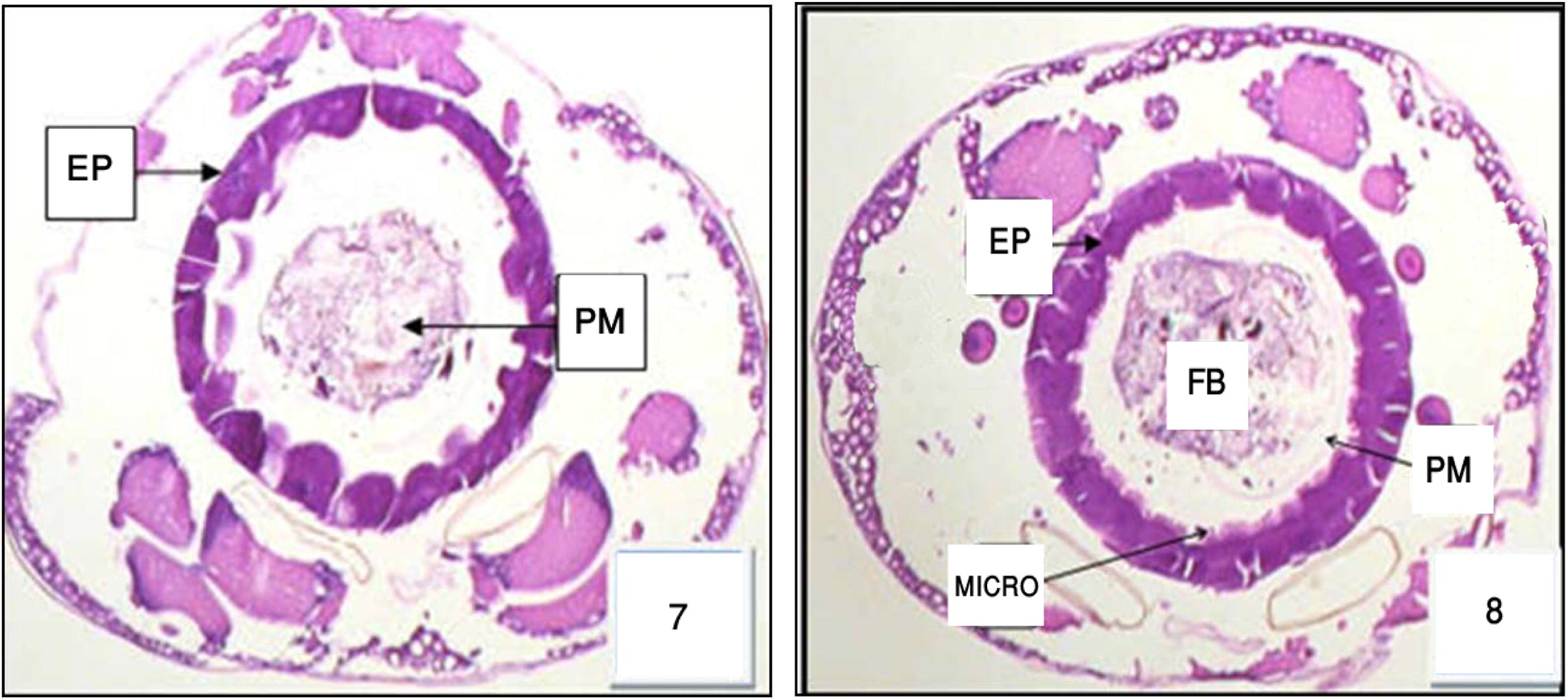
Cross section in the anterior and posterior region of midgut in 3rd Cx. quinquefasciatus larval instar treated with LC50 of M. azedarach extract, showing the effect after 24 h of exposure. Epithelial cells (EP) and food bolous (FB) and basement membrane (PM). 400×.
3.4 After 48 h of treatment
Most of the epithelial cells degenerated and vacuolated. Histopathological data reveal differential toxic effects of M. azedarach between the two different regions of the midgut epithelium according to the period of exposure. The major cytopathological alteration was large vacuoles of different sizes and containing broken membranes, at the apical side of the epithelial cells, and extensive cellular microvillar disruption. (Figs. 9 and 10 and ultrastructural sections Figs. 20 and 21).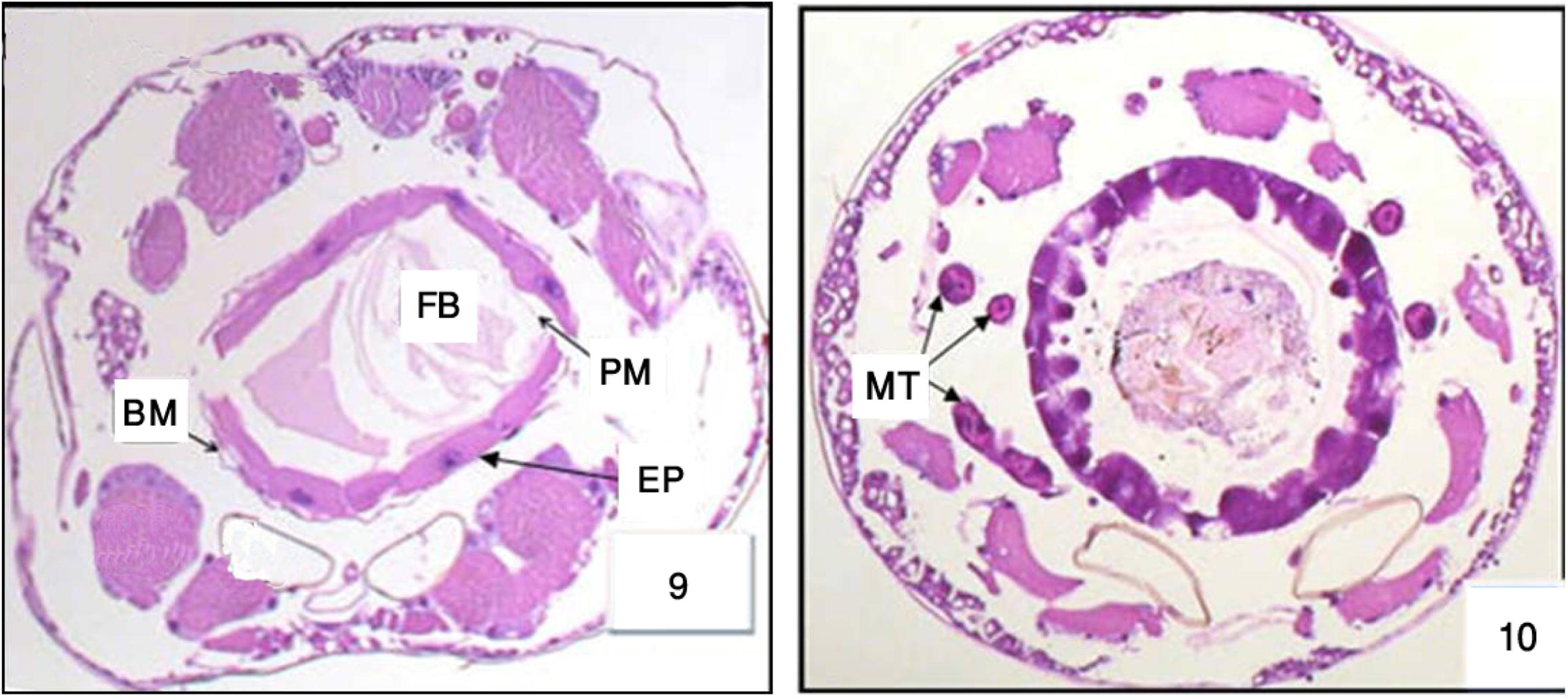
Cross section in the anterior and posterior regions of midgut in 3rd Cx. quinquefasciatus larval instar treated with LC50 of M. azedarach extract, showing the effect after 48 h of exposure. Epithelial cells (EP), peritrophic membrane (PM), food bolous (FB), malpighian tubules (MT) and basement membrane (BM). 400×.
3.4.1 Gastric caecae
The histopathological effect of Melia in gastric caeca region was studied. The choice of this region is justified by the fact that it is directly in contact with toxic element (azadirachtin) of melia compounds. The untreated larvae of Cx. quinquefasqiatus gastric caecum showed a well-preserved layer of epithelial cells. The ovoid nuclei are located in the center of the cell. One observes a regularly microvilli border in the gastric lumen (Fig. 11) for the treated larvae. After 6 h the signs of intoxication began on the level of caecum gastric. The histology of Cx. quinquefasciatus larvae showed on the level of this region morphological and serious damage of the epithelial columnar cells (Fig. 12). After 12 h some cells appear slightly hypertrophied with a perceptible beginning of vacuolization at the apical level. These vacuoles invaded the cells. Sometimes, we noted an enlargement of intercellular spaces (Fig. 13). After 24 h, the Epithelial cells of the gastric ceacae start to burst and we noted a cytoplasmic rejection of cells material that mixed with food column (Fig. 14). After 48 h, some cells degenerated and showed beaches of lysis (Fig. 15). These cells are very advanced in their infection.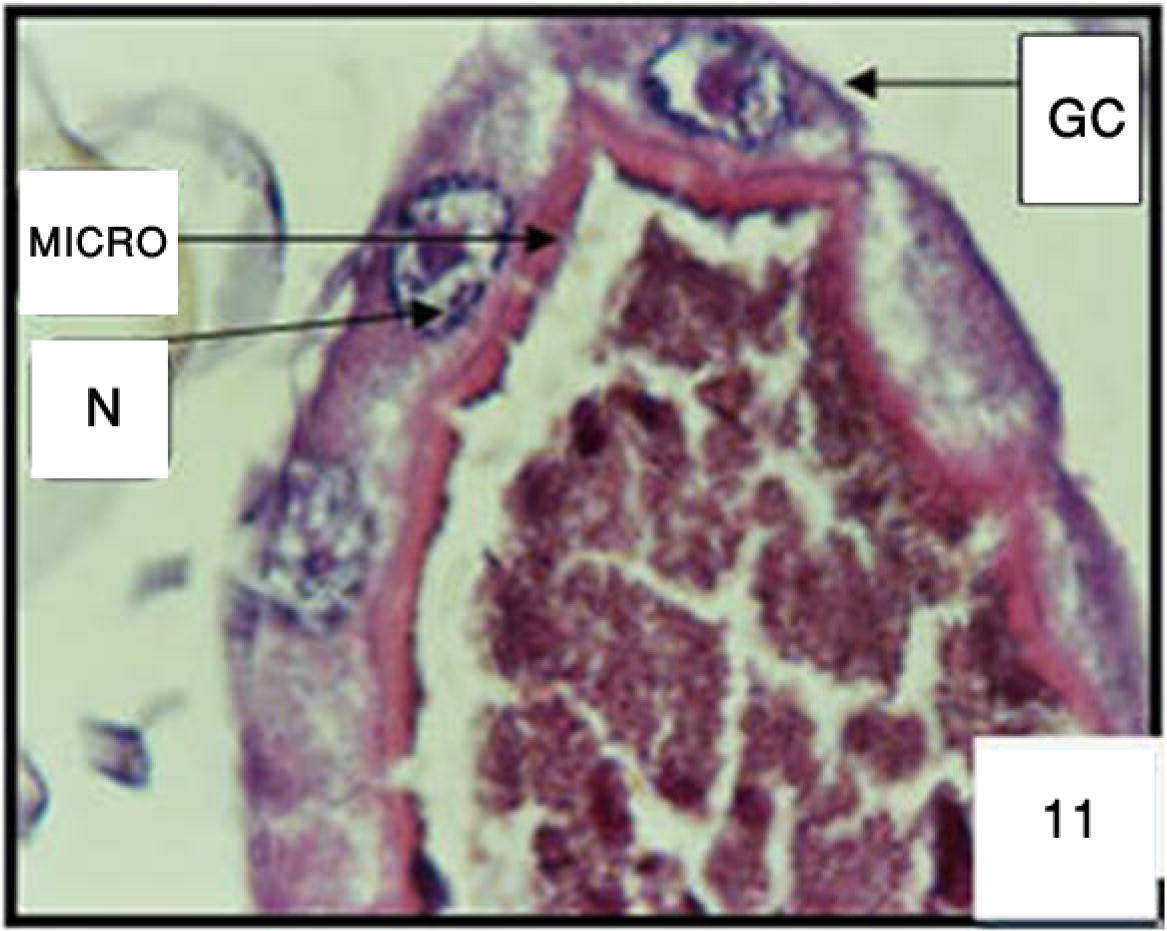
Cross section in gastric caeca (GC) of 3rd larval instar of Cx. quinquefasciatus untreated. Microvilli (MICRO) and nucleus (N). 400×.
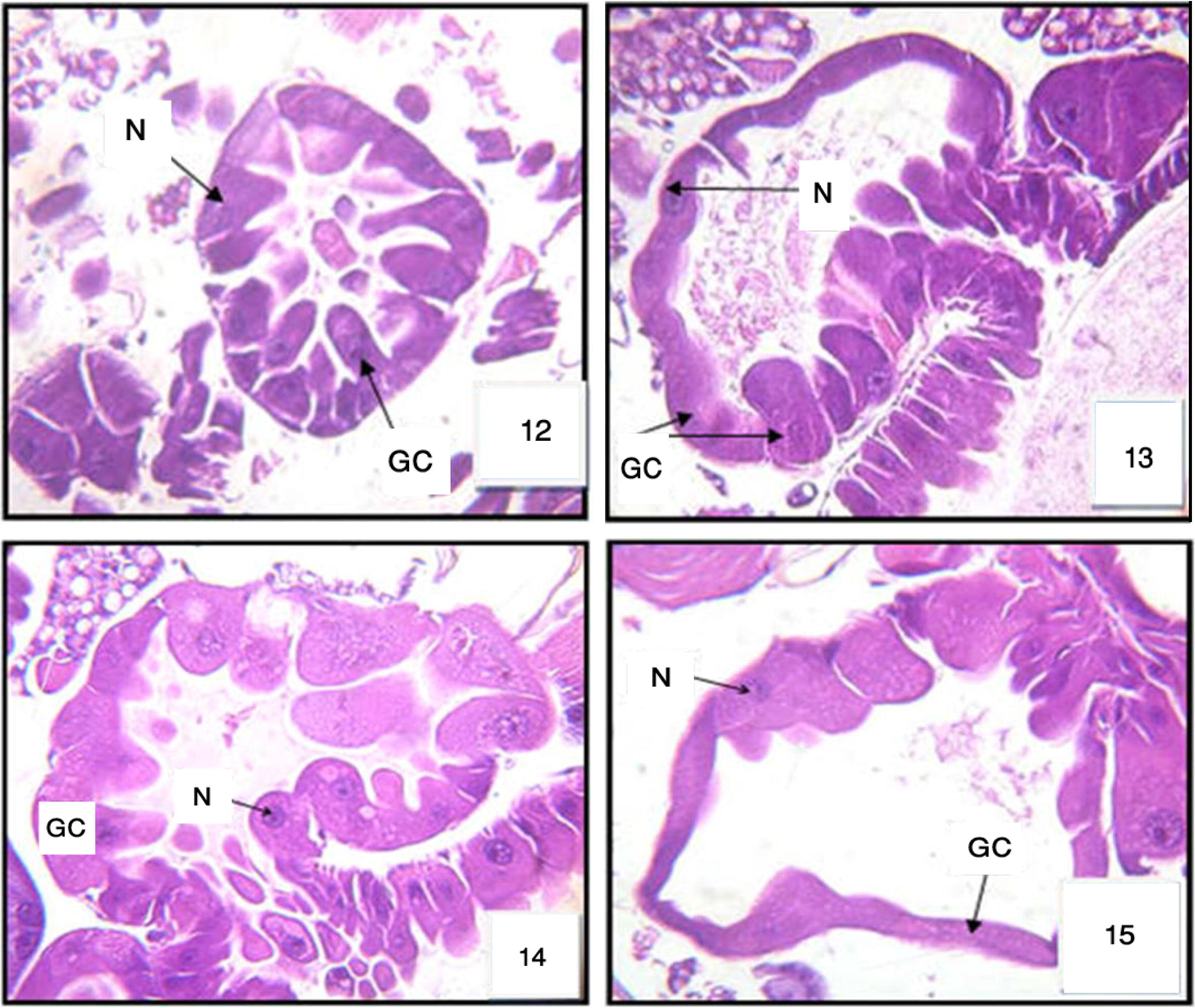
Cross section in gastric caeca (GC) of 3rd larval instar of Cx. quinquefasciatus treated with LC50 of M. azedarach after 6 h, after 12 h: Fig. 13, after 24 h: Fig. 14, and after 48 h: Fig. 15). Nucleus (N). 400×.
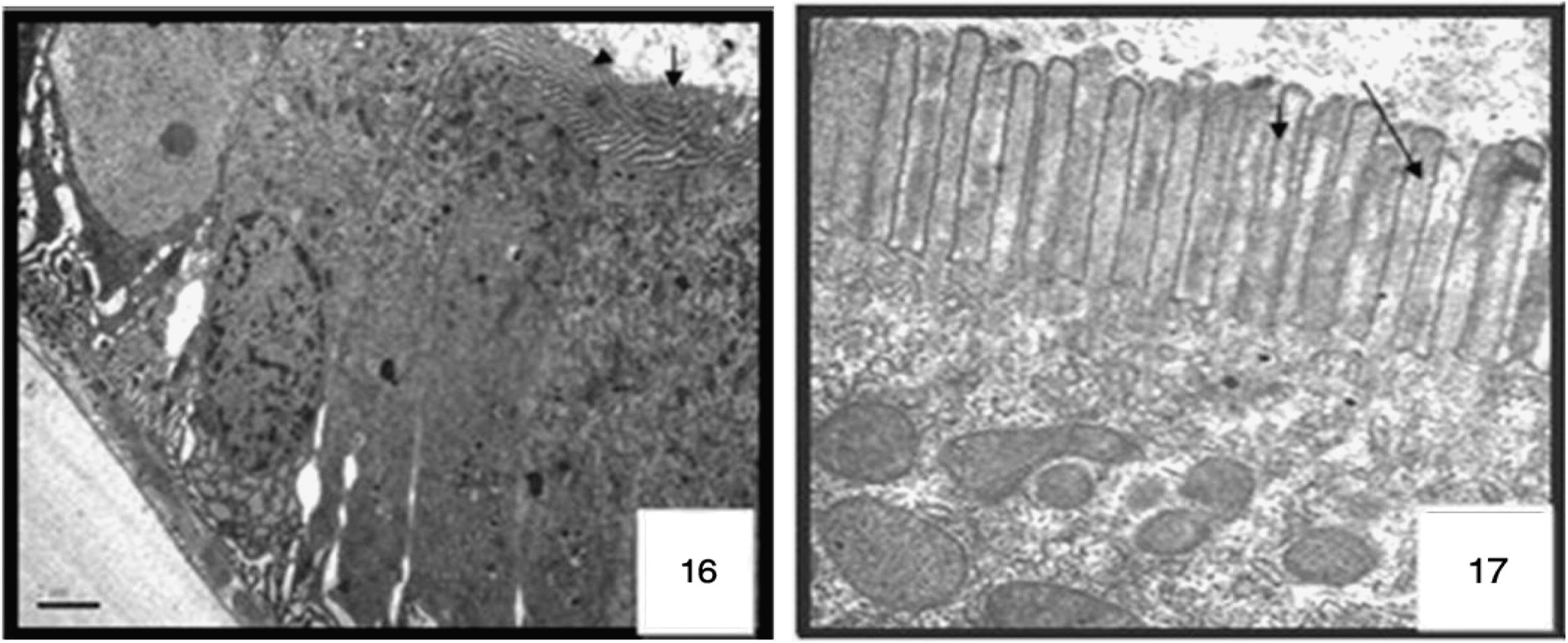
Longitudinal section of a columnar cell of the midgut in control larvae of Cx. quinquefasciatus. Transmission electron micrograph, microvilli ARROW. 4000×.
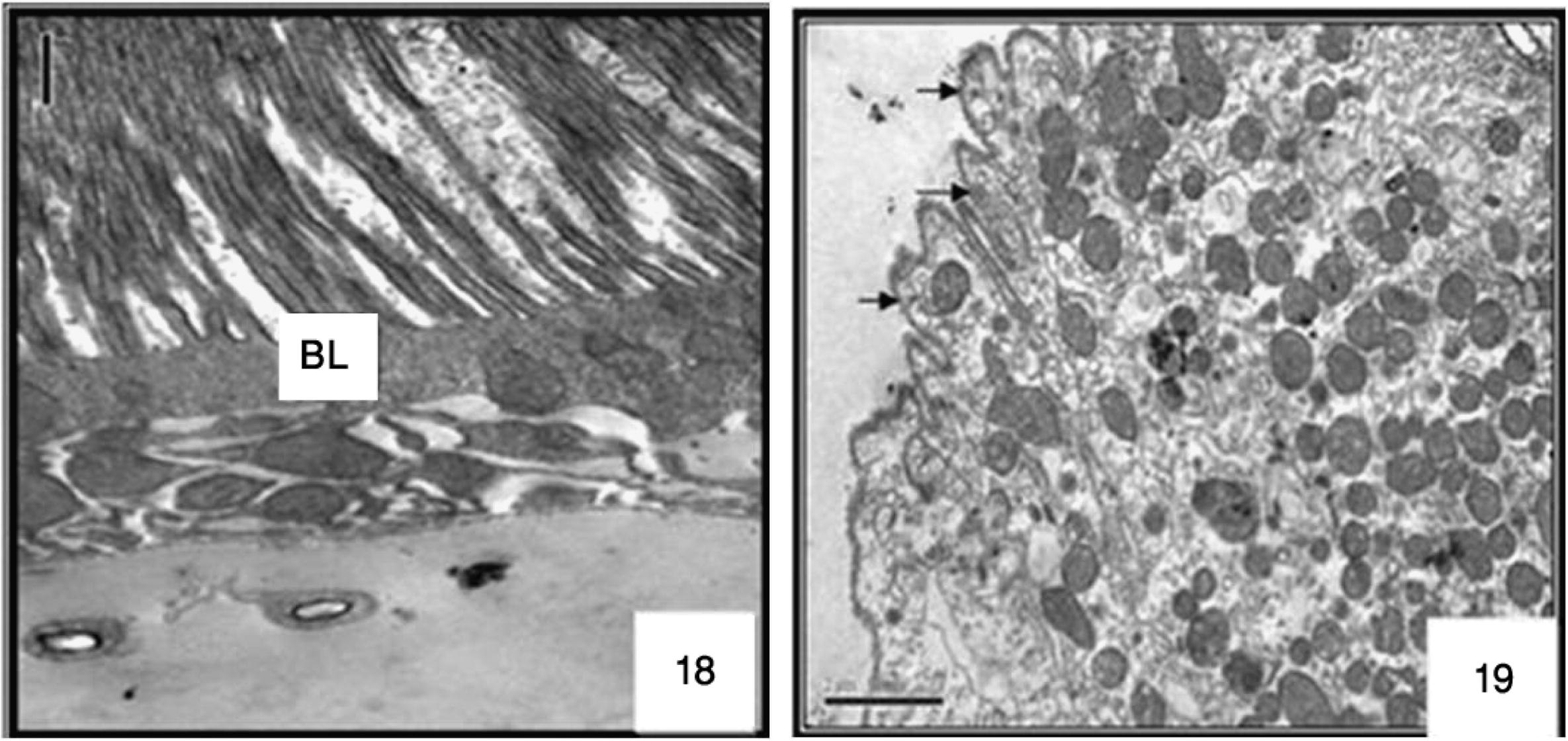
Ultrastructure of cells at 24 h after M. azedarach treatment larvae of Cx. quinquefasciatus. Transmission electron micrograph, destroyed MICROVILLI ARROW. 4000×.
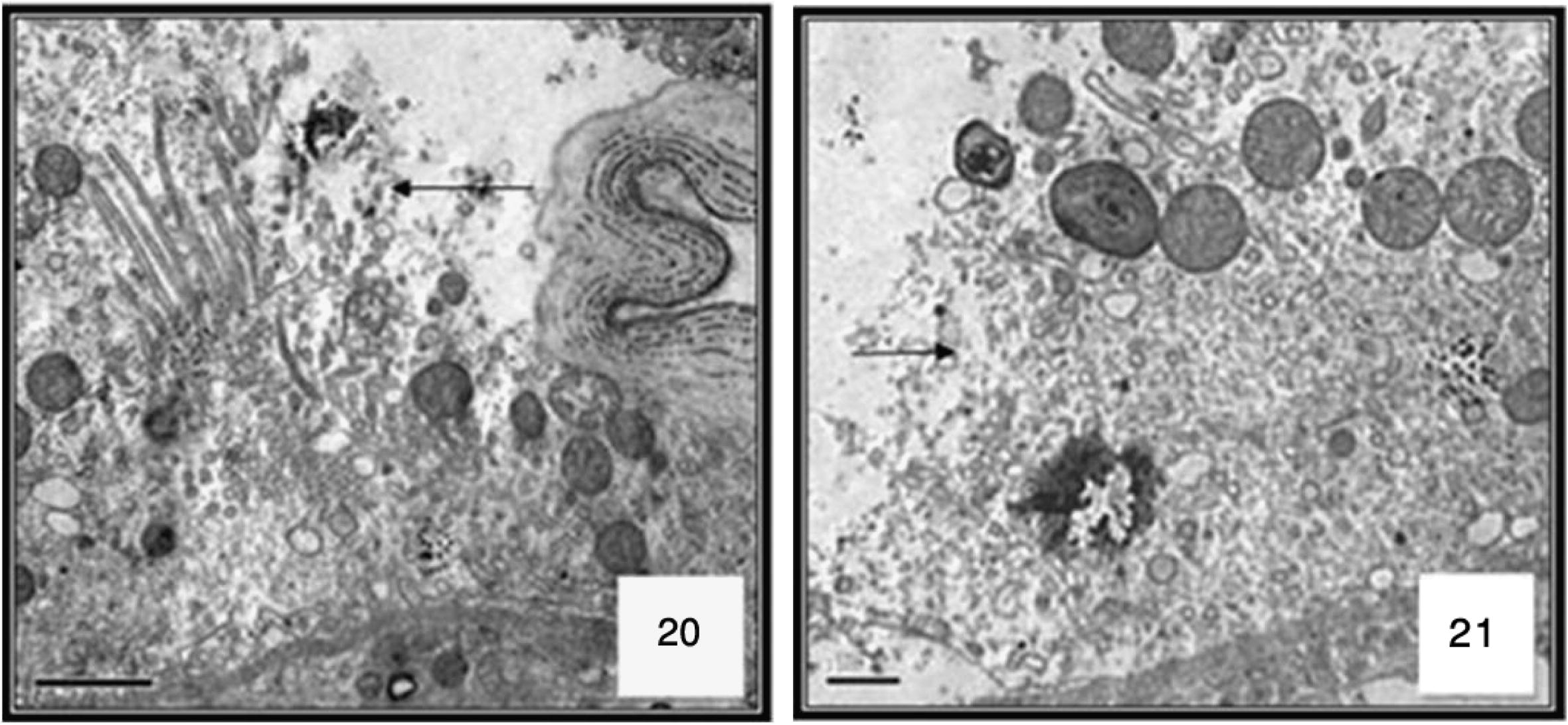
Ultrastructure of cells at 48 h after M. azedarach treatment larvae of Cx. quinquefasciatus. Transmission electron micrograph. 4000×. Cell disruption arrow.
4 Discussion
It is clearly proved that crude or partially purified plant extracts are less expensive and highly efficacious for the control of mosquitoes rather than the purified compounds or extracts (Jang et al., 2002; Cavalcanti et al., 2004). The plant tested in the present study is reported to be eco-friendly and are not toxic to vertebrates (Al-Sharook et al., 1991). In this study, the results showed that the ethanolic extract of M. azedarach exhibited hig degree of blocking the development by induction of great mortality of larvae. Concerning the insectiostatic or insecticidal action of M. azedarach, its leaves and fruits significantly reduced the growth of Spodoptera littoralis (Lepidoptera: Noctuidae) (Schmidt et al., 1998). Lymnaea cubensis (Pulmonata: Lymnaeidae), a Cuban intermediate host for fasciolosis, is lethally affected by the extracts of M. azedarach seeds (Perez et al., 1998). Anonymous (1999) described that leaves of M. azederach caused 54%, 62% and 75% %mortality of Callosobruchus chinensis at the rate of 0.5%, 1.0% and 2.0% (w/w) on the 12th day. But in this experiment M. azederach showed strong effective results. It showed high mortality after 24 and 48 h of treatment. The results of this study indicate the plant-based compounds such as limonoids (compounds present in the Meliaceae plant family seed) may be effective alternative to conventional synthetic insecticides for the control of Cx. quinquefasciatus. Undoubtedly, plant-derived toxicants are a valuable source of potential insecticides. These and other naturally occurring insecticides may play a more prominent role in mosquito control programs in the future (Mordue and Blackwell, 1993). The results of this study will contribute to a great reduction in the application of synthetic insecticides, which in turn increase the opportunity for natural control of various medicinally important pests by botanical pesticides. Since these are often active against a limited number of species including specific target insects, less expensive, easily biodegradable to non-toxic products, and potentially suitable for use in mosquito control programme (Alkofahi et al., 1989), they could lead to the development of new classes of possible safer insect control agents. Changes were seen in the anterior and posterior regions of the midgut, included separation of the epithelial cells from the basement membrane with damage of the peritrophic membrane. The mixing of the gut contents with the haemolymph caused the larval mortality. The border of midgut showed a striated appearance due to the presence of the microvilli which line the inner edge of the epithelial cells. These microvilli enhanced the rate of absorption (DeRobertis et al., 1965). The gut apical portion of columnar cells was swollen and sometimes, distinct elongations protruded into its lumen as a bulbous eversion section showed vacuolated cytoplasm, cells were dislodged, sloughed and detached from each other. The most obvious ultra structural change noted in the epithelium of the midgut was the disruption of the microvilli, thus giving the midgut a vacuolization appearance, this coincides with the observations of Sutter and Raun (1967) in Ostrinia nubilalis who suggested that the enzymatic activity of the vegetative rods is responsible for the disruption of microvilli.
The observed histopathological effects of the ethanolic extract of M. azedarach on the midgut of Cx. quinquefasciatus larvae were in agreement with the results obtained by Hamouda et al. (1996), Hussin and Shoukry(1997) and Assar and El-Sobky (2003) on Cx. pipiens. Hamouda et al. (1996) stated that the midgut of Cx. pipiens treated with Artemisia judaica was affected, the epithelial layer was vacuolated, swollen cells, masses of cellular material appeared in the lumen and finally the epithelium lost their normal appearance. Also, they found that larvae treated with Anagallis arvensis showed a rupture of the cell wall and destruction of the peritrophic membrane. Assar and El-Sobky (2003) observed that the water extract of Eichhornia crassipes, revealed drastic effect on larval midgut as the brush border and some of the epithelial cells were apically degenerated after 48 h and after 72 h, most of the epithelial cells completely degenerated and vacuolated.
The results of the present study may encourage further researches on using simple and inexpensive application methods for controlling mosquitoes in their breeding sites.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Ent.. 1925;18(1925):265-267.
- [Google Scholar]
- Search for new pesticides from higher plants. In: Arnason J.T., Philogene B.J.R., Morand P., eds. Insecticides of Plant Origin. Washington, DC: American Chemical Society; 1989. p. :25-43.
- [Google Scholar]
- Insect growth inhibitors from two tropical meliaceae. Effect of crude seed extracts on mosquito larvae. J. Appl. Ent.. 1991;111:425-430.
- [Google Scholar]
- Anonymous, 1999. Test of botanicals for their pesticidal properties against bruchuid weevil Callosobruchus chinensis (Coleoptera: Bruchidae) in lentil. Annual Technical Report. Khumaltar, Kathmandu. R.N. Chopra, R.L. Badhwar.
- Biological and histopatological studies of some plant extracts on larvae of Culex pipiens (Diptera: Culicidae) J. Egypt. Soc. Parasitol.. 2003;33(1):189-200.
- [Google Scholar]
- Carpinella, M.C., Defago, M.T., Valladares, G., Palacios, S.M., 2003. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. Agric. Food Chem. 15 (51), 369–674.
- Cavalcanti, E.S.B., de Morais, S.M., Ashley, A.L.M., William, P.S.E., 2004. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Memo´ rias do Instituto Oswaldo Cruz 99, 541–544.
- Efeitos de extratos de pimenta-preta sobre larvas de Culex (Culex) quinquefasciatus Say (Diptera: Culicidae) An. Soc. Entomol. Brasil. 1994;23:13-18.
- [Google Scholar]
- Ultrastructural midgut events in Culicidae larvae fed with Bacillus sphaericus 2297 spore/crystal complex. Annales de l’Institut Pasteur/Microbiol.. 1987;138:471-484.
- [Google Scholar]
- Chockalingam, S., Thenmozhi, S., Nalina Sundari, M.S. 1990. Larvicidal activity of different products against mosquito larvae. Ind. J. Environ. Biol. 41, 101–104.
- Larvicidal, adulticidal and repellent effects of Kaempferia galanga. Southeast Asian J. Trop. Med. Public Health. 1999;30:470-476.
- [Google Scholar]
- The biology of mosquitoes.Development, Nutrition and Reproduction. Vol vol. 1. London: Chapman & Hall; 1992.
- Ultrastructural effects of the Bacillus sphaericus mosquito larvicidal toxin on cultured mosquito cells. J. Invertebr. Pathol.. 1987;50:213-220.
- [Google Scholar]
- DeRobertis E.D., Nowinski A.A., Seaz F., 1965. Cell Biology, 4th ed. Saunders, Philadelphia, PA, p. 117.
- Laboratory study on the mosquito larvicidal properties of leaf and seed extract of plant Agave americana. J. Trop. Med. Hyg.. 1987;90:79-82.
- [Google Scholar]
- Insecticidal and synergistic activity of Atriplex halimus L. Extracts. J. Egypt Soc. Parasitol.. 1998;28:191-196.
- [Google Scholar]
- Toxic and growth retarding effects of tree plant extracts on Culex pipiens larvae (Diptera: Culicidae) Phytother. Res.. 1999;13:388-392.
- [Google Scholar]
- Probit Analysis (third ed.). London, UK: Cambridge University Press; 1971. p. 38
- Insect antifeedant and growth-regulating activities of salannin and other C-seco limonoids from neem oil in relation to azadirachtin. J. Chem. Ecol.. 1996;22:1453-1456.
- [Google Scholar]
- Toxicity and histopathological effects of Artemisia Judaic and Anagallis arvensis extracts on Culex pipiens larvae. J. Egypt. Ger. Soc. Zool.. 1996;20(E):43-60.
- [Google Scholar]
- Hussin, K.T., Shoukry, F.I., 1997. Toxicological and histopathological studies of certain plant fixed oils on Culex pipiens larvae. Ain Shams Sci. Bull. 35, 287–305.
- Larvicidal activity of Brazilian plants against Aedes Aegypti and Culex pipiens pallens (Diptera: Culicidae) Agric. Chem. Biotechnol.. 2002;44:23-26.
- [Google Scholar]
- Limonoids from Citrus reticulata and their moult inhibiting activity in mosquito Culex quinquefasciatus larvae. Phytochemistry. 1997;44:843-846.
- [Google Scholar]
- Antifeedant activity of fruit and seed extracts of Melia azedarach and Azadirachta indica on larvae of Sesamia nonagrioides. Phytoparasitica. 2000;28:311-319.
- [Google Scholar]
- Larvicidal and synergistic activity of plant extracts for mosquito control. Ind. J. Med. Res.. 1985;82:1-19.
- [Google Scholar]
- Larvicidal activity of Commiphora molmol against Culex pipiens and Aedes caspius larvae. J. Egypt Soc. Parasitol.. 2000;30:101-115.
- [Google Scholar]
- Use of neem oil as a mosquito repellent in tribal villages of Mandla District, Madhya Pradesh. Indian J. Malariol.. 1995;32:99-103.
- [Google Scholar]
- Antipupational effect of neem oil and neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston) J. Ent. Res.. 1996;20:137-139.
- [Google Scholar]
- Effects of plant extracts on fecundity and fertility of mosquitoes. J. Appl. Entomol.. 2001;125:31-35.
- [Google Scholar]
- Nasiruddin, M., Mordue, A.J., 1993. The effect of azadirachtin on the midgut histology of the Locust, Shistocerca gregaria and Locusta migratoria.Tissue & Cell, 25(6), 875–884.
- Insecticidal and insect repellent activity of essential oils of Tridax procumbens and Cyathocline lyrata. Fitoterapia. 1988;59:211-219.
- [Google Scholar]
- Perez, M.P., Fernandez, L.D., Guirado, O.A.A., Capote, R.V., Aguilar, G.G., 1998. Actividade molusquicida del paraiso (Melia azedarach L.) (Meliaceae) sobre Lymnaea cubensis,molusco vector de Fasciolosis. Revista de Sau´de Pu´blica 32, 262–266.
- Screening for larvicidal activity of ten carminative plants. Southeast Asian J. Trop. Med. Public Health. 1998;29:660-662.
- [Google Scholar]
- Rey, D., Pautou, Marie-Paule, Meyran, Jean-Claude, 1999. Histopathological effects of tannic acid on the midgut epithelium of some aquatic diptera larvae. J. Invertebr. Pathol. 73, 173–181.
- Saxena, R.C., Epino, P.B., Cheng-Wen, T., Puma, B.C., 1984. Neem, chinaberry and custard apple: antifeedant and insecticidal effects of seed oils on leafhopper and planthopper pests of rice. In: Proceedings of 2nd International Neem Conference, Rauischholzhausen, Germany, pp. 403–412.
- Effect of Melia azedarach fruit extract on juvenile hormone titer and protein content in the hemolymph of two species of noctuid lepidopteran larvae (Insecta: Lepidoptera: Noctuidae) Phytoparasitica. 1998;26:283-291.
- [Google Scholar]
- Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Ann. Rev. Ent.. 1990;35:271-297.
- [Google Scholar]
- Senthil Nathan, S., Kalaivani, K., Murugan, K., Chung, P.G., 2005. The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guene´e), the rice leaffolder. Pest. Biochem. Physiol. 81, 113–122.
- Immunocytochemical localization of the Bacillus sphaericus binary toxin components in Culex quinquefasciatus (Diptera: Culicidae) larvae midgut. Pestic. Biochem. Physiol.. 2003;77:138-146.
- [Google Scholar]
- An electron microscope study of the toxic action of Bacillus sphaericus in Culex quinquefasciatus larvae. J. Invertebr. Pathol.. 1988;52:237-247.
- [Google Scholar]
- Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae) J. Am. Mosq. Control Assoc.. 1998;14:204-209.
- [Google Scholar]
- Sutter, G., Raun, E.S., 1967. Histopathology of European corn borer larvae, treated with bacillus thuringiensis. J. Invertbr. Path. 9, 90–103.
- World Health Organization, 2005. Ref: WHO/CDS/WHOPES/GCDPP/2005.13: Guidelines for Laboratory and Field Testing of Mosquito Larvicides.
- Effect of some crude and azadirachtin-enriched Neem (Azadirachta indica) seed kernel extracts on larvae of Aedes aegypti. Entomol. Exp. Appl.. 1984;35:11-16.
- [Google Scholar]







