Translate this page into:
Fascioliasis prevalences among animals and human in Upper Egypt
*Corresponding author. Address: Life Sciences Department, Faculty of Science, King Khalid University, 9004 Abha, Saudi Arabia. Mobile: +966 555835233 abdelnasser@lycos.com (Abdel-Nasser A. Hussein)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Coprological surveys on animal and human fascioliasis were performed in Qena, a governorate located in Upper Egypt. Animal and human stool samples were collected from four localities: Abu-Tesht, Qena, Armant, and Isna. A total of 297 stool samples from different animals were examined. These samples are (105 cows, 163 buffaloes and 29 sheep). The overall prevalence was 30.3% and including 28.6% in cows, 33.7% in buffaloes, and 17.2% in sheep. These differences in prevalences between definitive hosts were not significant. However, prevalences were significantly different among localities, these values being lower in Qena (7.6%) and Armant (18.2%) than in Abu-Tesht (54.7%) and Isna (42.6%). No fasciolid egg was found in human examined stool samples from the same localities.
Keywords
Survey
Prevalence
Fascioliasis
Human
Animal
Upper Egypt
1 Introduction
Fascioliasis is a worldwide disease caused by liver flukes of the genus Fasciola (see reviews in Dalton (1999) and Hurtrez-Boussès et al. (2001)). It has long been considered an important veterinary problem, particularly in regions with intensive sheep or cattle production, leading to high economic losses (Torgerson and Claxton, 1999). It is now also recognized as a human disease of large public health importance (Mas-Coma, 2004), including high pathogenicity (Mas-Coma et al., 2000) and presenting from hypo- to hyperendemic areas (Mas-Coma, 2005; Mas-Coma et al., 1999a,b, 2005). Fascioliasis is an emerging or re-emerging disease in several parts of the world, mainly in South America, Africa and Asia (Mas-Coma, 2004).
In Egypt, several reasons make the situation of fascioliasis particularly interesting. Indeed (1) fascioliasis is probably present for a very long period, since its symptoms have been identified in cattle represented in Egyptian tombs (Esteban et al., 2003) and the presence of liver fluke fragments in an Egyptian mummy (David, 1997). (2) Moreover, high infestation levels have been described in livestock (Soliman, 1998). Such infestation rates induce important economic problems. The annual loss in milk and meat due to fascioliasis being estimated to be 30% (Haseeb et al., 2002). (3) Since 1980, the number of human cases has dramatically increased especially in the Nile Delta region, which is meso- to hyperendemic (Esteban et al., 2003). It is estimated that 830,000 humans are infected and that 27 millions of persons are exposed to the risk of infection (WHO, 1995). Moreover, fascioliasis appears frequently associated to other detrimental diseases, especially schistosomiasis (Esteban et al., 2003). Therefore, fascioliasis is an emerging disease which is already considered a serious health problem in Egypt (Curtale et al., 2000, 2005). (4) In Egypt, both Fasciola gigantica and F. hepatica coexist (Esteban et al., 1998). (5) Fasciola gigantica, with a tropical and subtropical distribution (Spithill et al., 1999), is considered the endogenous fasciolid species in Egypt. On the contrary, F. hepatica, which has a wide range, is originating from Europe where it was introduced to other continents through the importation of domestic animals (Mas-Coma and Bargues, 1997) and similarly to Egypt (Soliman, 1998). For these reasons, and in order to envisage policies of health care, surveys on the distribution of fascioliasis in Egypt are required. Most of the studies have been conducted on the Nile Delta region (Lower Egypt), where several human cases have been described (Haseeb et al., 2002; Esteban et al., 2003). Contrarily, the situation is poorly known in Upper Egypt. Nevertheless, in this region, agricultural and pastoral activities are widely developed and safety conditions are often low which lead to potential risks of contamination. The aim of this study is to survey fascioliasis among domestic animals and humans in Qena Governorate, in Upper Egypt.
2 Materials and methods
2.1 Study area
Qena governorate is located in Upper Egypt, about 600 km at the South of Cairo. It extends over a distance of 240 km (see Fig. 1), and is bordered on the North by Sohag Governorate and on the South by Asswan. The total area of Qena governorate is 10,800 km2, but only 1740 km2 are inhabited, along the Nile Valley. Among the 2.8 millions people living in this governorate, 900,000 persons inhabit urban areas, whereas the rest of the population lives in rural zones.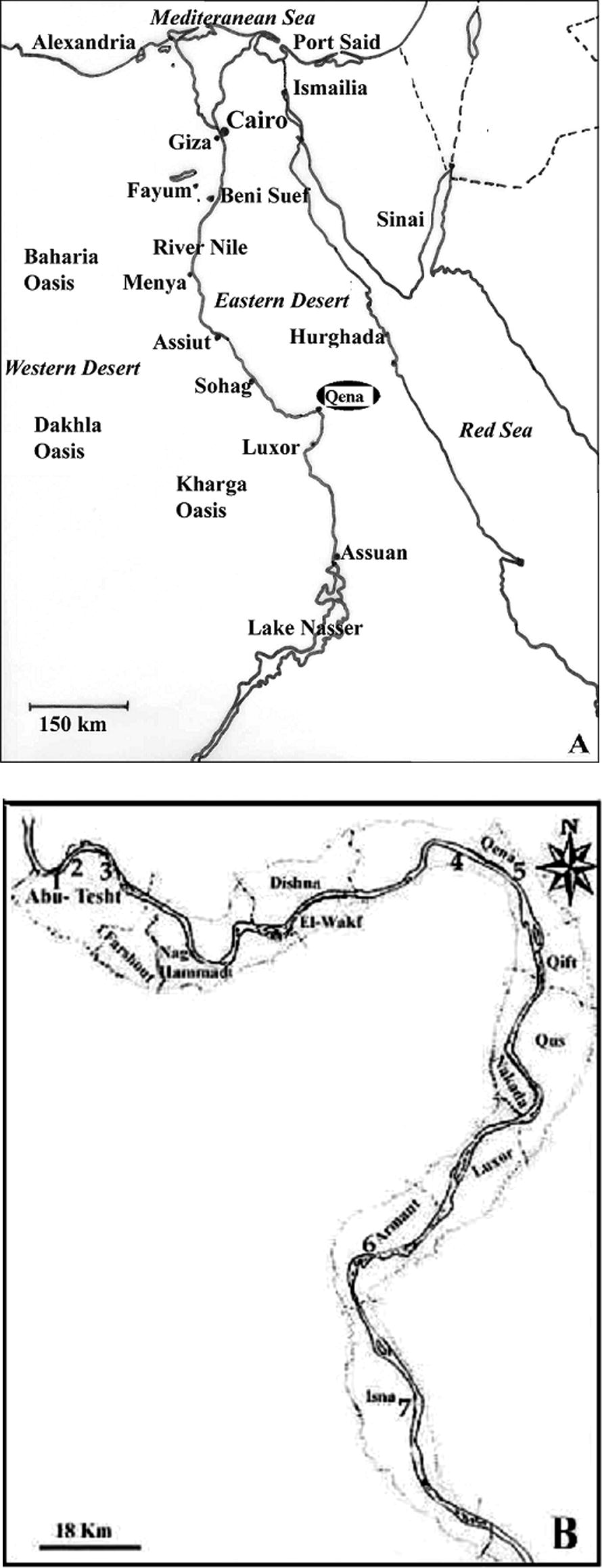
Map of Qena governorate (A), indicating the four studied localities (Abu-Tesht, Qena, Armant and Isna) and the location of localities surveyed for human samples (B). Numbers indicate the localities in which human stools have been sampled (N = sample size). 1: Nag. Ahmed Bekheet (N = 26), 2: Nag. El-Rebba (N = 21), 3: Abu-Tesht (N = 19), 4: El-Tramsa (N = 28), 5: Qena (N = 13), 6: Gezeret Armant El-Hate (N = 17), 7: El-Shaghab (N = 28).
Samples were collected from the four following localities: Abu-Tesht, Qena, Armant and Isna (see Fig. 1 for the exact location of sampling sites). Abu-Tesht and Isna are rural areas, in which the main activities are agriculture and pastoralism, whereas Armant combines agricultural and industrial activities and Qena city is the most urbanized.
2.2 Sampling
In each of the studied localities, cattle, buffaloes and sheep were chosen randomly in different herds. For each selected animal, fresh stool was collected immediately after excretion or from the rectum using gloved fingers. A total of 297samples were examined: 105 from cows (13 males and 92 females), 163 from buffaloes (18 males and 145 females), and 29 from sheep (4 males and 25 females). Sample sizes per locality and per host species are given in Table 1. Each sample was immediately stored in a small sterile plastic cup and transported to the laboratory. Regarding human fascioliasis, the survey was performed on children 8–17-years-old, in some villages in Qena Governorate. Samples were taken from the four studied localities but sampling effort was more important in Abu-Tesht locality, where most of the people are farmers raising livestock. Localities surveyed for human samples are mentioned in Fig. 1. A total of 150 human stool samples were collected, including 29 females and 121 males. One stool sample for each person was collected and personal data (name, sex, and age) were noted on delivery of the container. Faecal samples were transported to the laboratory within 1–3 hours of collection. The survey was carried out after informed consent was obtained from the local authorities of the villages, as well as by all participants.
Locality
Host species
Buffalo
Cow
Sheep
Total
Abu-Tisht
79
30
8
117
Qena
38
30
11
79
Armant
8
26
10
44
Isna
38
19
0
57
Total
163
105
29
297
2.3 Coprological Analysis
In the laboratory, stools were concentrated by the sedimentation method (Little and Yaeger, 1985) and then examined microscopically, in order to look for liver fluke eggs. No attempt was made to discriminate between F. hepatica and F. gigantica, because of the impossibility to differentiate the two species at egg level (Marcilla et al., 2002). Thus, both are referred as Fasciola spp., after Esteban et al. (2003). Similarly, since egg production shows a marked seasonality, we did not estimate intensities (i.e. parasite burden per host). Therefore, the only data obtained was the prevalence, i.e. the proportion of infected individuals among the total examined.
2.4 Statistical analysis
We analyzed the effect of locality and definitive host species on prevalences. Analyses were performed with the GLIM computer program (The Royal Statistical Society, London) (NAG, 1986; Crawley, 1993), with stepwise deletion of variables without significant effect, after correction for overdispersion. Since the dependent variable is a proportion, we used a binomial error model (Crawley, 1993).
2.5 Institutional ethical review procedure
The surveys were carried out after informed consent was obtained from the local authorities in the villages, as well as by all participants.
3 Results
Eggs of Fasciola spp. were found in 90 (30.3%) of 297 animal stool samples examined. Prevalences per species and per locality were given in Fig. 2. Generally the highest infection rate was found in Abu-Tesht, followed by Isna and Armmnt. The lowest infection rate was detected in Qena locality. Although prevalence values differ between host species (28.6% in 105 cows, 33.7% in buffaloes, and 17.2% in sheep), the difference was not significant (F2,13 = 0.217, NS) after controlling the effects of the locality. However, prevalences appeared to be significantly different among localities (F3,15 = 11.60, p < 0.01; interaction species * locality: F5,8 = 3.01, NS). Regarding host species, buffaloas have been possessed the higest prevalence (54.9%), followed by cows (35.4%) and the lowest prevalence was found among sheep (9.8%). Table 1 shows the prevalence of Fasciola eggs in each examined host in the different four localities.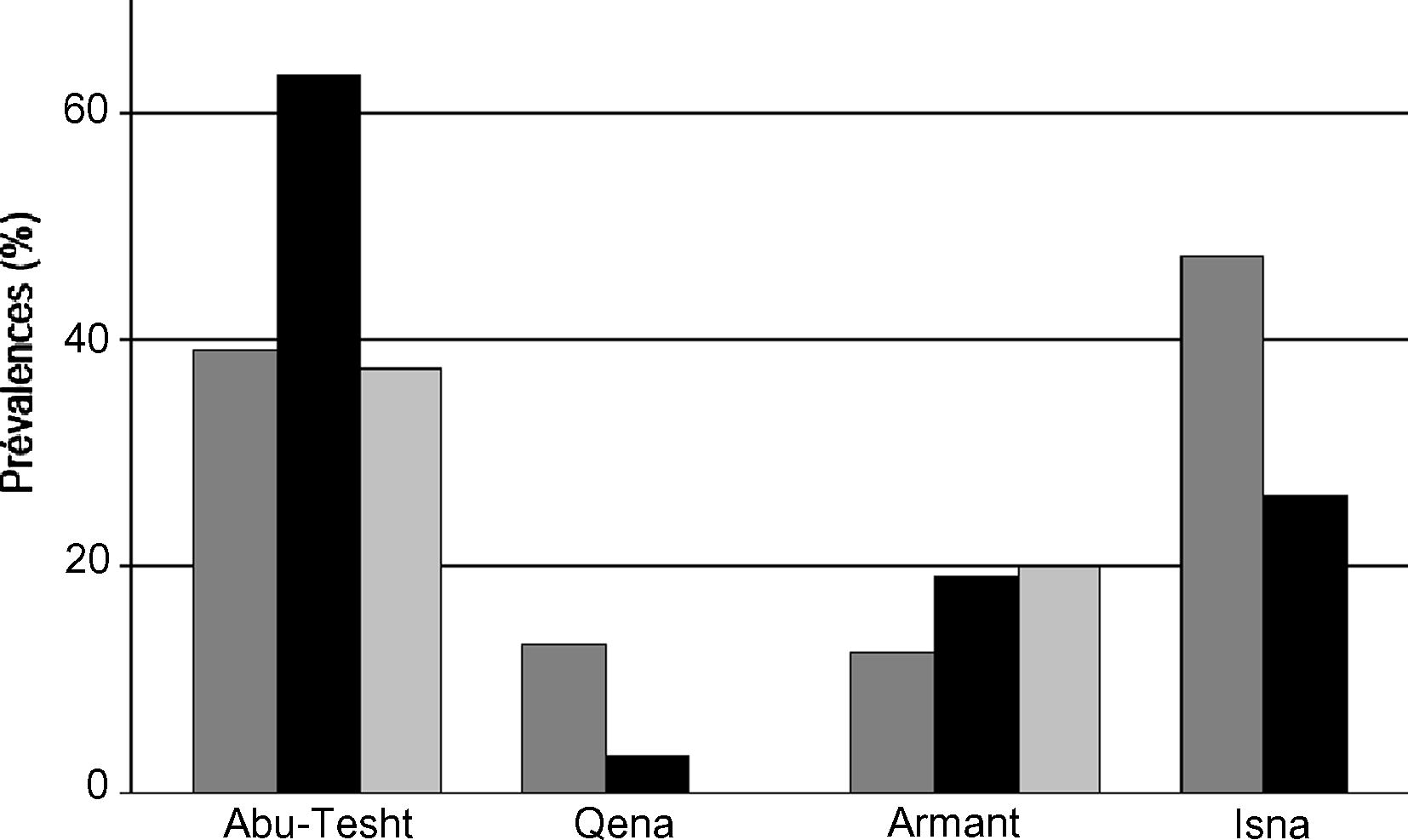
Prevalence of eggs of Fasciola spp. in coprological samples of buffalo (dark grey), cow (black) and sheep (light grey bars) in four localities of Qena governorate (Egypt).
No fasciolid egg was found in the 150 human stools samples examined.
4 Discussion
Although coprological methods probably underestimate the number of cases (Mas-Coma et al., 1999b), our study reveals high prevalences (30.3% among the whole sample) in livestock in the Qena Governorate, Upper Egypt. The values obtained are much higher than global surveys for the whole country (Abdel-Aziz, 1993; Haridy et al., 1999) which showed 5.3%, 3.5%, 1.2% and 0.5% in cattle, buffaloes, sheep and goats and 3.5%, 1.5% and 2.0% for cattle, buffaloes, sheep and goats, respectively. However, our results are consistent with similar studies conducted along the Nile valley, especially in Lower Egypt, where relatively high prevalences have been reported. Indeed, in the Northern part of the country, in Abis II village, 44%, 27%, 25% and 59% were detected in cattle, buffaloes, sheep and goats, respectively (Allam, 1992). These data are in agreement with previous studies conducted in Alexandria (El-Sherif et al., 1959) and in El Gharbia Governorate (Zaki et al., 1965) which revealed prevalences of 22% and 15.9% in cattle and 13% and 10.9% in buffaloes. Similarly, in Assiut Governorate (located about 400 km at the South from Cairo), 30.3%, 24.3%, 26.2% and 6.4% prevalences were detected in cattle, buffaloes, sheep and goats, respectively (Monib, 1977). Such high prevalences have important economic consequences and can be considered as a big problem for the development of the regions. In Qena Governorate, the prevalence was slightly lower for sheep (17.2%) than for cows (28.6%) and buffaloes (33.7%). Such differences might be explained by the fact that F. gigantica is very abundant in Egypt (Spithill et al., 1999) and that buffalo is considered as the preferred definitive host of F. gigantica (Yadav et al., 1999; Chen et al., 2000), followed by cow, another large size cattle species more appropriate for F. gigantica development than sheep (Hammond and Sewell, 1974). However, the differences were not significant after controlling for the effects of site, suggesting that the three different species may have similar probabilities of infestation.
The present survey revealed significant differences in prevalences between studied localities. Indeed, it was found that lower infestation rates in Qena (7.6%) and Armant localities (18.2%) than in Abu-Tesht (54.7%) and Isna localities (42.6%). Qena city harbours the main city of the governorate (Qena), and pastoralism is a marginal activity in this locality, whereas the other localities are dominated by rural areas.
The result obtained in the human survey fully agreed with the results of the large Qena governorate survey previously performed in 1992 (Curtale et al., 1998). In this study, no fascioliasis infection was found in stool samples from a total of 2657 children, 2–12-years-old, originating from the 12 administrative localities of the governorate of Qena. These results in humans contrast with the high prevalences observed in domestic animals in Qena, which are similar to livestock prevalences found in Nile Delta areas where human fascioliasis is a great public health problem, as in Behera and Alexandria governorates (Esteban et al., 2003). Two main hypotheses might explain the different fascioliasis situations in humans between Upper and Lower Egypt, despite similar prevalences in animals. First, the species of potential lymnaeid snail vectors may differ between the two regions. Indeed, in Qena governorate the only suitable vector described is Radix natalensis (Hussein et al., 2005), the specific intermediate host for F. gigantica in Africa (Mas-Coma and Bargues, 1997). On the contrary, many other lymnaeid species, potentially vectors of F. hepatica, are additionally known in Lower Egypt (Brown, 1994). Second, the commercial livestock usual procedures in Egypt may rise to the detection of parasitized animals in given places whereas in fact those animals were originally contaminated elsewhere. Indeed, in this country, animal importation from other countries (both European and African) (Soliman, 1998) and livestock transportation and exchange within the inland are common (F.M. Haridy, 1999). Such practices may also favour the detection of animals infected by fluke populations including both imported and autochthonous parasite specimens. It must be taken into account that fasciolid flukes have a long adult life span in the definitive host, as for instance of up to 1 year in cattle and 13 years in sheep (Mas-Coma and Bargues, 1997). The above-mentioned two aspects may also be in the background of the pronounced prevalence differences detected among localities and between definitive hosts.
Although no human case of fascioliasis had been detected in this study, as well as in a previous large survey, our study highlights the fact that prevalences of Fasciola spp. are high in livestock. Therefore, Qena governorate can be considered as an area at risk for human populations and health care policies have to take such presence of fascioliasis into account.
Acknowledgement
This work is dedicated to the memory of late Prof. Dr. Ismael M. Hassan, a good father and teacher, Zoology Department, Faculty of Science, South Valley University, Qena, Egypt. Also my appreciation to Dr. Abdel-Azeem Abdel-Baki, King Saud University, Saudi Arabia, for his great help in publishing this manuscript.
References
- Abdel-Aziz, N.M., 1993. Incidence and public health importance of fascioliasis in slaughtered animals in Assiut Province. M.Sc. Thesis, Faculty of Veterinary Medicine, Assiut University, Egypt.
- Allam, A.F.M.B., 1992. Studies on the Lymnaea–Fasciola (host–parasite) relationships in Abis area. Ph.D. Thesis, High Institute of Public Health, Alexandria University, Egypt.
- Freshwater Snails of Africa and Their Medical Importance (second ed.). London: Taylor and Francis Ltd.; 1994. 608pp
- Blood eicosanoids and immune indices during fasciolosis in water buffaloes. Parasitol. Int.. 2000;49:273-278.
- [Google Scholar]
- GLIM for Ecologists. Methods in Ecology. Oxford: Blackwell Science Publications; 1993.
- Knowledge, perceptions and behaviour of mothers toward intestinal helminths in Upper Egypt: implications for control. Health Policy Plann.. 1998;13:423-432.
- [Google Scholar]
- Human fascioliasis, an emerging public health problem in the Nile Delta, Egypt. Res. Rev. Parasitol.. 2000;60:129-134.
- [Google Scholar]
- Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Trans. Roy. Soc. Trop. Med. Hyg.. 2005;99:599-609.
- [Google Scholar]
- Fasciolosis. Wallingford, Oxon, UK: CAB International Publishing; 1999.
- Disease in Egyptian mummies; the contribution of new technologies. The Lancet. 1997;349:1760-1763.
- [Google Scholar]
- The incidence of parasitic infestation among the farm animals of Faculty of Agriculture, University of Egypt. Vet. Med. Assoc.. 1959;19:19-21.
- [Google Scholar]
- Geographical distribution, diagnosis and treatment of human fascioliasis: a review. Res. Rev. Parasitol.. 1998;58:13-42.
- [Google Scholar]
- Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am. J. Trop. Med. Hyg.. 2003;69:429-437.
- [Google Scholar]
- The pathogenic effect of experimental infections with Fasciola gigantica in cattle. Br. Vet. J.. 1974;130:453-464.
- [Google Scholar]
- Fascioliasis an increasing zoonotic disease in Egypt. J. Egypt Soc. Parasitol.. 1999;29:35-48.
- [Google Scholar]
- Dynamics of host–parasite interactions: the example of population biology of the liver fluke (Fasciola hepatica) Microbes Infect.. 2001;3:841-849.
- [Google Scholar]
- Trematode larval stages infecting Radix natalansis (Gastropoda: Lymnaeidae) in Qena Governorate, Egypt, with special reference to fasciolid cercariae. Res. Rev. Parasitol.. 2005;66(1–4):69-74.
- [Google Scholar]
- Diagnostic materials and methods. In: Beaver P.C., Jung R.C., eds. Animal Agents and Vectors of Human Disease (fifth ed.). Philadelphia: Lea and Febiger; 1985. p. :247-256.
- [Google Scholar]
- A PCR-RFLP assay for the distinction between Fasciola hepatica and F. gigantica. Mol. Cell. Probe. 2002;16:327-333.
- [Google Scholar]
- Human fascioliasis. In: Cotruvo J.A., Dufour A., Rees G., Bartram J., Carr R., Cliver D.O., Craun G.F., Fayer R., Gannon V.P.J., eds. World Health Organization (WHO), Waterborne Zoonoses: Identification, Causes and Control. London, UK: JIWA Publishing; 2004. p. :305-322.
- [Google Scholar]
- Epidemiology of fascioliasis in human endemic areas. J. Helminth.. 2005;79:207-216.
- [Google Scholar]
- Epidemiology of human fascioliasis: a review and proposed new classification. Bull. World Health Organ.. 1999;77:340-346.
- [Google Scholar]
- Human fasciolosis. In: Dalton J.P., ed. Fasciolosis. Wallingford, Oxon, UK: CAB International Publishing; 1999. p. :411-434.
- [Google Scholar]
- Mas-Coma, S., Bargues, M.D., Marty, A.M., Neafie, R.C., 2000. Hepatic trematodiases, in: Meyers, W.M., Neafie, R.C., Marty, A.M., Wear, D.J. (Eds.), Pathology of Infectious Diseases, Helminthiases, vol. 1, Armed Forces Institute of Pathology and American Registry of Pathology, Washington, DC, pp. 69–92.
- Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol.. 2005;35:1255-1278.
- [Google Scholar]
- Monib, M.E.M., 1977. Study on some helminth parasites of ruminants in Assiut Governorate. M.Sc. Thesis, Faculty of Veterinary Medicine, Assiut University, Egypt.
- The Generalised Interactive System. Release 3.77. London: The Royal Statistical Society; 1986.
- Control of veterinary fascioliasis. In: Angelico M., Rocchi G., eds. Infectious Diseases and Public Health. A Research and Clinical Update. Philadelphia, L’Aquila: Balaban Publishers; 1998. p. :334-346.
- [Google Scholar]
- Fasciola gigantica: epidemiology, control, immunology and molecular biology. In: Dalton J.P., ed. Fasciolosis. Wallingford, Oxon, UK: CAB International Publishing; 1999. p. :465-525.
- [Google Scholar]
- Epidemiology and control. In: Dalton J.P., ed. Fasciolosis. Wallingford, Oxon, UK: CAB International Publishing; 1999. p. :113-149.
- [Google Scholar]
- World Health Organization, 1995. Control of Foodborne Trematode Infections. WHO Technical Report Series. World Health Organization, Geneva, No. 849, pp. 1–157.
- Primary experimental infection of riverine buffaloes with Fasciola gigantica. Vet. Parasitol.. 1999;82:285-296.
- [Google Scholar]
- Zaki, H., El-Refaii, A.H., Saliman, M.K., 1965. Hematology of normal cattle and buffaloes and those infected with Fasciola gigantica. In: Proceedings of the Sixth Annual Arabian Veterinary Congress, Cairo, p. 263.







