Translate this page into:
Seasonality and prevalence of Microsporidium sp. infecting lizard fish, Saurida undosquamis from the Arab Gulf
*Corresponding author. Address: Zoology Department, College of Science, King Saud University, P.O. Box 12455, Riyadh 11451, Saudi Arabia azema1@yahoo.com (A.S. Abdel-Baki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
During the present study seasonal prevalence of Microsporidium sp. infecting lizard fish, Saurida undosquamis were reported. Infection was reported as whitish cysts in the skeletal muscles. The mature spores were ovoid to pyriform with large vacuole at their ends. Sixty out of 200 fish were infected (30%). The highest infection was in autumn (64%) while the lowest one was in winter (14%) and no great difference was found between summer (24%) and spring (18%).
Keywords
Microspora
Lizard fish
Seasonal variation
1 Introduction
Microspora is one of the most important protozoan widespread, affecting most of invertebrates and vertebrates addition to humans (Lom, 2002). It is characterized by unicellular eukaryotic microorganisms living as obligate intracellular parasites, commonly infecting fishes, insects, crustaceans, and other invertebrate and vertebrate groups from different geographical areas (Lom and Dykova, 1992; Sprague et al., 1992; Larsson, 1999; Lom, 2002). Microsporidia infecting the musculature of commercially important fish species have a negative impact on fish product (Yokoyama et al., 2002). Such microsporidians species belong to the genera Heterosporis, Pleistophora, Kabatana and the collective group Microsporidium (Dykova, 1995). Some of Microsporidium species forms elongated whitish nodules in the musculature which causes liquefaction of muscle fibers resulting in a characteristic concave body surface and, in extreme circumstances, may result in the death of the host fish (Yokoyama et al., 2002).
There is no knowledge about microsporidiosis in the ichthyological fauna of Arabian Gulf. This study shed light on the prevalence and seasonal variation of one of microsporean species infecting the lizard fish, Saurida undosquamis from the Arabian Gulf.
2 Materials and methods
Two hundred specimens of marine fish S. undosquamis (Richardson, 1848) (Synodontidae, Harpadontinae) were collected from the fish market at Damam through monthly visits. Macroscopic examination of different organs was carried out for detection of any visible microsporean cysts. Squash preparations from gills, intestine, kidney, spleen and liver were examined microscopically. The recorded microsporean cysts were measured then squashed for studying the spore morphology. Measurements of fresh spores were taken using calibrated ocular micrometer on Olympus microscope.
For the cyst structure studies, the cysts were excised and fixed in 3% glutaraldehyde in 0.2 M sodium cacodylate buffer (pH 7.2) at 4 °C for 24 h. After washing overnight in the same buffer at 4 °C and post-fixation in 2% osmium tetroxide in the same buffer and temperature for 3 h, the fragments were dehydrated through a graded ethanol ascending series, followed by propylene oxide (three changes of 2 h each) and embedded in Epon (12 h in each change). Semi-thin sections were stained with methylene blue.
3 Results
The infection was observed as numerous macroscopic, whitish and spindle-shaped cysts through out the skeletal muscle (Fig. 1). The number of cysts reached up to 20 cysts per the infected fish. The cyst was up to 3–8 mm in size. In wet mounts, fresh spores were mostly ovoid to pyriform in shape and possessed a large vacuole at the posterior end (Fig. 2). They were 4 (3.5–4.5) × 2 (1.5–2.5) μm in size. Histological observations showed that parasite cysts were encapsulated by a host produced fibrous membrane (Fig. 3). No liquefaction of muscle fibers was observed. The interior of the mature cyst was occupied by many developmental stages in many chamber-like compartments separated from each other by septa (Fig. 4).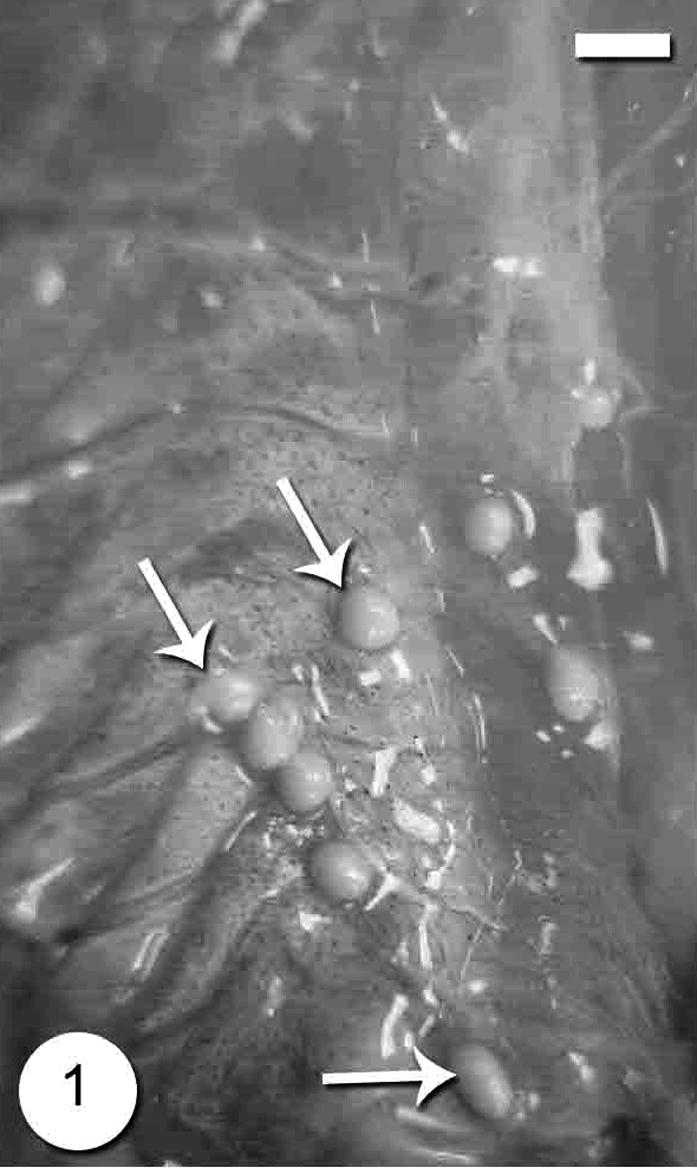
Photograph of the Microsporidium sp. cysts in the muscles of Microsporidium sp. infecting lizard fish, Saurida undosquamis. Scale-bar = 10 μm.
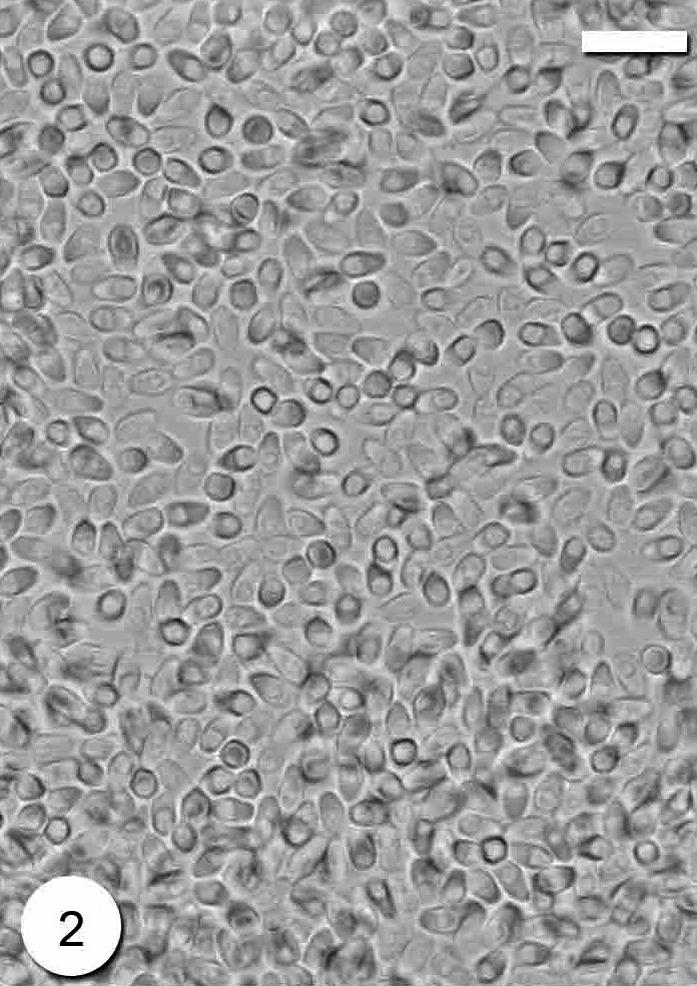
Photomicrograph of Microsporidium sp. fresh spores. Scale-bar = 10 μm.
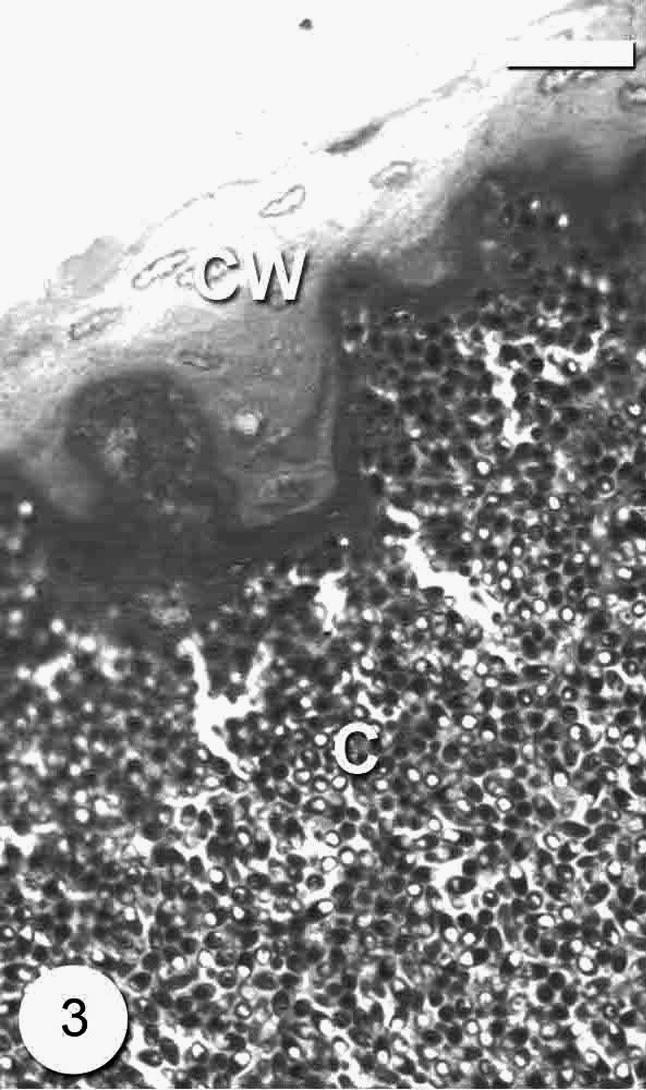
Photomicrograph of semi-thin section through the Microsporidium sp. cyst (C) surrounded with thick cyst wall (CW). Bar = 10 μm.
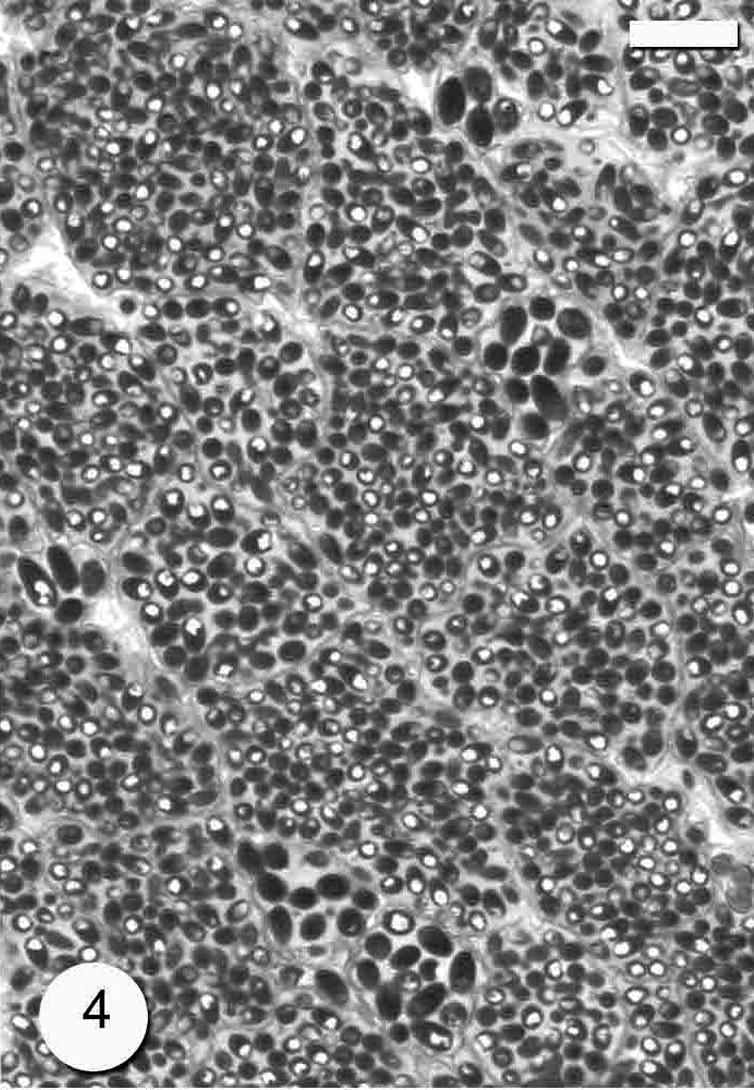
Photomicrograph of semi-thin section through the Microsporidium sp. cyst which divided by many septa (arrows). Bar = 10 μm.
3.1 Prevalence and seasonal variation
The overall prevalence was 30% (60/200). The highest infection rate was in autumn (64%) followed by summer (24%) and winter (18%), and the lowest one was in winter (14%) (Fig. 5).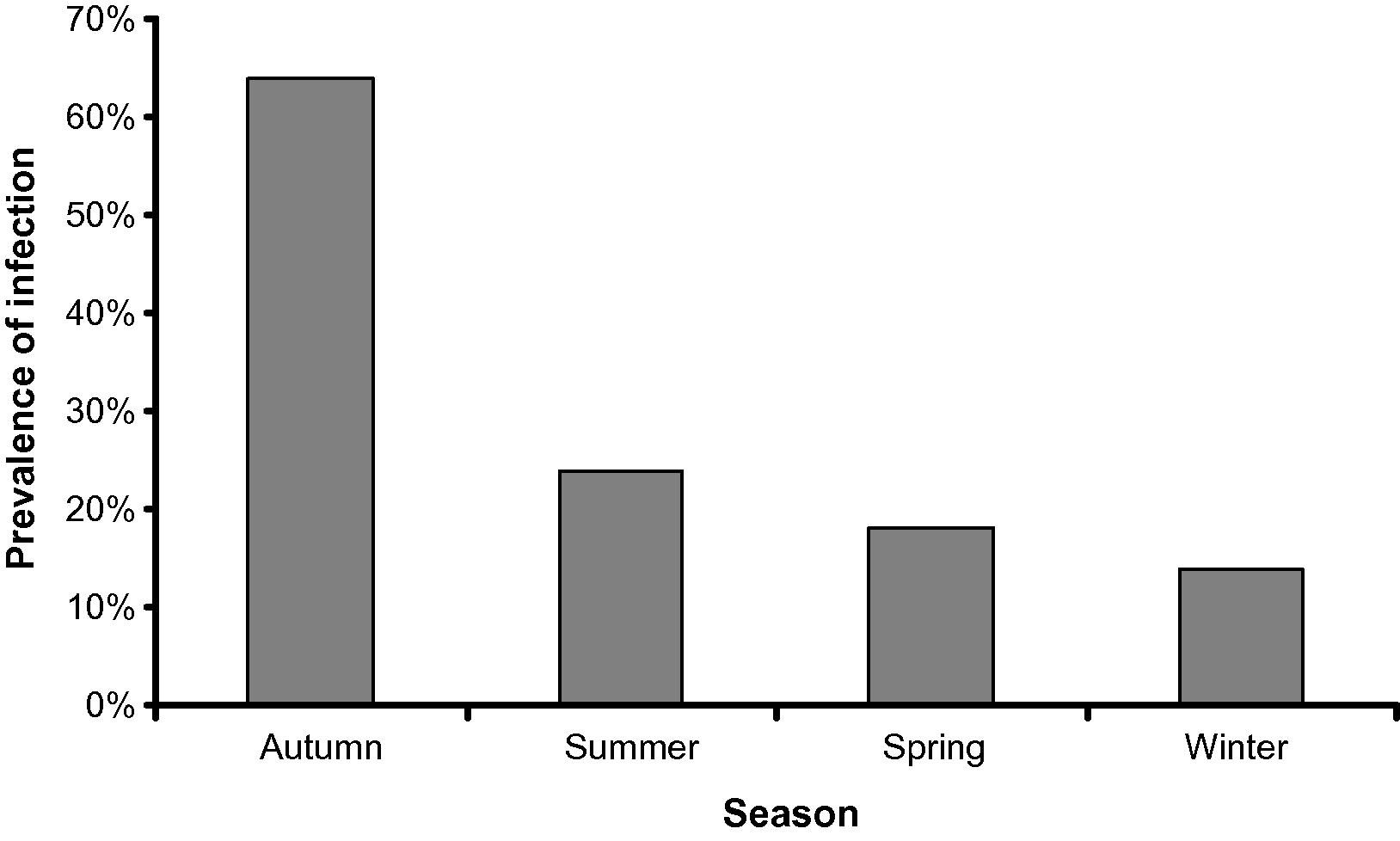
The relationship between the season and the prevalence of infection of Saurida undosquamis with Microspora sp.
4 Discussion
Microsporidian taxonomy is traditionally based on the spore morphology and the pre-spore development, principally at the ultrastructural level (Yokoyama et al., 2002). Although transmission electron microscopy is still essential to microsporidia systematic, a number of discrepancies between phylogenies based on ultrastructure and molecular analyses have been revealed (Nilsen, 2000). The future classification of microsporean parasites will require a combination of morphological and molecular data (Yokoyama et al., 2002). Therefore, the conclusive identification of the present microsporean species will precluded for further ultrastructure and molecular studies. Accordingly, the present species will allocate to the collective group Microsporidium till further studies.
There are very few previous reports that were conducted extensively on the seasonal prevalence of microsporidia. All these studies were carried out on the microsporean parasites of insects (Araujo-Coutinho et al., 2004) and human (Conteas et al., 1998). No information about the seasonality and prevalence rates of fish microsporeans. Our study revealed that the average of the prevalence was 30% and the highest prevalence in autumn and no great differences among the other seasons. A similar result was reported by Lass and Ebert (2006). Cycles in parasite prevalence are commonly observed through rarely understood epidemiological phenomena (Hosseini et al., 2004; Lass and Ebert, 2006). Cyclic patterns of the parasite may due to the climatic conditions, food conditions, host behavior, and host immunity (Grassly et al., 2005).
In conclusion this study provided with information on the seasonal prevalence of microsporean species infecting the lizard fish in Arab Gulf.
Acknowledgement
The study was supported by College of Science, Research Center, Project No. (Zoo/2009/20).
References
- Seasonality and prevalence rates of microsporidia in Simulium pertinax (Diptera: Simuliidae) larvae in the region of Serra dos Orgaos Rio de Janeiro, Brazil. J. Invertebr. Pathol.. 2004;85:188-191.
- [Google Scholar]
- Examination of the prevalence and seasonal variation of intestinal microsporidiosis in the stools of persons with chronic diarrhea and human immunodeficiency virus infection. Am. J. Trop. Med. Hyg.. 1998;58(5):559-561.
- [Google Scholar]
- Phylum microspora. In: Woo P.T.K., ed. Fish Diseases and Disorders. In: Woo P.T.K., ed. Protozoan and Metazoan Infections. Vol vol. 1. Cambridge, UK: CAB International; 1995. p. :149-179.
- [Google Scholar]
- Host immunity and synchronized epidemics of syphilis across the United States. Nature. 2005;433:417-421.
- [Google Scholar]
- Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. Roy. Soc. B. 2004;271:2569-2577.
- [Google Scholar]
- Apparent seasonality of parasite dynamics: analysis of cyclic prevalence patterns. Proc. Roy. Soc. B. 2006;273:199-206.
- [Google Scholar]
- A catalogue of described genera and species of microsporidians parasitic in fish. Syst. Parasitol.. 2002;53:81-99.
- [Google Scholar]
- Protozoan Parasites of Fishes. Amsterdam: Elsevier Science Publishers; 1992. 315pp
- Small subunit ribosomal DNA phylogeny of Microsporidia with particular reference to genera that infect fish. J. Parasitol.. 2000;86:128-133.
- [Google Scholar]
- Occurrence of a new Microsporidium in the skeletal muscle of the flying fish Cypselurus pinnatibarbatus japonicus (Exocoetidae) from Yakushima, Japan. Folia Parasitol.. 2002;49(1):9-15.
- [Google Scholar]







